7-Fluoroindole
CAS Number 387-44-0
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials, MonomersA fluorinated indole
Used as a synthesis intermediate for APIs, dyes and polyindoles in application of drug discovery, OLEDs, DSSCs and batteries
Specifications | MSDS | Literature and Reviews
7-Fluoroindole (CAS number 387-44-0) is a fluorinated heterocyclic compound derived from indole, a benzene fused pyrrole. 7-Fluoroindole has proven effective on supressing swarming motility, protease activity and the production of a polymeric matrix in Pseudomonas aeruginosa, an opportunistic human pathogen. The anti-virulence of 7-fluoroindole is based on decreasing the production of quorum sensing related phenotypes. 7-Fluoroindole is also used in 19F NMR study on the binding affinity to R67 dihydrofolate reductase (an enzyme confers resistance to antibacterial drug trimethoprim).
7-Fluoroindole can be used to synthesize polyindole by oxidative polymerization. Polyindole is a conducting polymer that has been applied in batteries (polyindole/LiClO4/Li with discharge capacity of 116–124 mAh/g at current density of 0.5 A/m2).
Multiple functional groups
For facile synthesis
Fluorinated indole building block
For drug discovery, OLEDs, and batteries
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 387-44-0 |
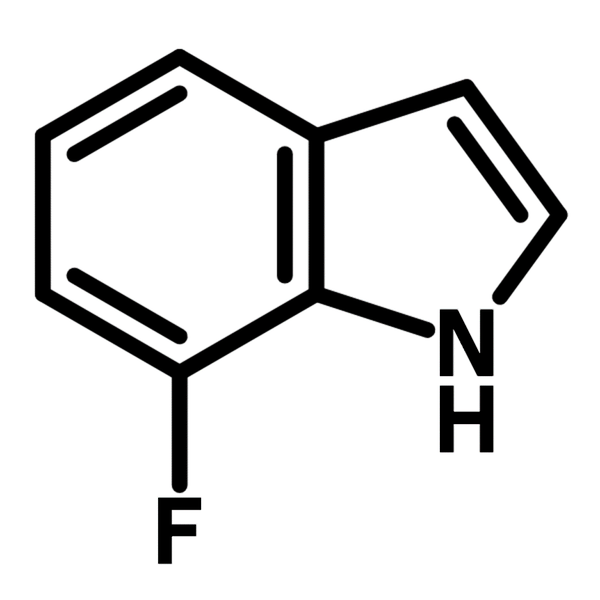
| Chemical Formula | C8H6FN |
| Full Name | 7-Fluoro-1H-indole |
| Molecular Weight | 135.14 g/mol |
| Synonyms | N/A |
| Classification / Family | Fluorinated building block, Heterocyclic building block, APIs, Batteries, OLEDs, DSSCs |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 60 °C – 65 °C |
| Appearance | Off-white powder |
MSDS Documentation
Literature and Reviews
-
7-fluoroindole as an antivirulence compound against Pseudomonas aeruginosa, J.-H. Lee et al., FEMS Microbiol. Lett., 329, 36–44(2012); DOI: 10.1111/j.1574-6968.2012.02500.x.
-
Differentiation of the binding of two ligands to a tetrameric protein with a single symmetric active site by 19F NMR, G. Fuente-Gómez et al., Protein Science, 30, 477–484(2021); DOI: 10.1002/pro.4007.
-
Mixed fluorotryptophan substitution at the same residue expand the versatility of 19F protein NMR spectroscopy, C. Kenward etal., Chem. Eur. J., 24, 3391–3396(2018); DOI: 10.1002/chem.201705638.