2,4-Difluoro-(1H-1,2,4-triazolyl)acetophenone
CAS Number 86404-63-9
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, MonomersA fluorinated triazole building block
A synthesis intermediate for APIs in drug discovery and bioimaging
Specifications | MSDS | Literature and Reviews
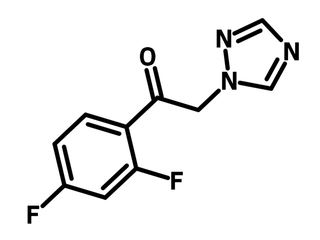
2,4-Difluoro-(1H-1,2,4-triazolyl)acetophenone (CAS number 86404-63-9) has a difluoroacetophenone that is attached to a triazole unit. 2,4-Difluoro-(1H-1,2,4-triazolyl)acetophenone is a molecular scaffold for triazole antifungal medications, for instance, fluconazole, voriconazole and efinaconazole. The ketone moiety in 2,4-difluoro-(1H-1,2,4-triazolyl)acetophenone allows various modifications via Grignard reaction and aldol reaction. Enantioselective additions are also sufficient with chiral prolines catalyzed aldol reaction or chiral auxiliaries assisted Grignard reaction.
With the addition of chromophores, 2,4-difluoro-(1H-1,2,4-triazolyl)acetophenone has been used to study the mechanism of antifungal activity in live Candida cells, with fluorescent microscopy.
Multiple functional groups
For facile synthesis
Fluorinated triazole building block
For drug discovery, medicinal chemistry, and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 86404-63-9 |
| Chemical Formula | C10H7F2N3O |
| Full Name | 2,4-Difluoro-(1H-1,2,4-triazolyl)acetophenone |
| Molecular Weight | 223.18 g/mol |
| Synonyms | 1-(2,4-Difluorobenzoylmethyl)-1H-1,2,4-triazole, 1-(2,4-Difluorophenyl)-2-(1H-1,2,4-triazol-1-yl)ethanone, Voriconazole Related Compound C, Fluconazole Impurity E |
| Classification / Family | Fluorinated building block, Heterocyclic building block, APIs, Bioimaging |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 103 – 107 °C |
| Appearance | Off-white powder |
MSDS Documentation
2,4-Difluoro-(1H-1,2,4-triazolyl)acetophenone MSDS Sheet
Literature and Reviews
-
Conazole, J. Heeres et al., Molecules, 15, 4129-4188(2010); DOI: 10.3390/molecules15064129.
-
Enantiomerically pure tertiary alcohols by TADDOL-assisted additions to ketones—or how to make a Grignard reagent enatioselective, B. Weber et al., Angew. Chem., Int. Ed., 31(1), 84–86(1992); DOI: 10.1002/anie.199200841.
-
Pharmacological significance of triazole scaffold, R. Kharb et al., J. Enzyme Inhib. Med. Chem., 26(1), 1-21(2011); DOI: 10.3109/14756360903524304.
