4-(Trifluoromethyl)-2-pyridone
CAS Number 50650-59-4
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials,A highly electron deficient pyridone derivative
Used as building block for the synthesis of key intermediates in the production of pharmaceuticals and drug discovery
Specifications | MSDS | Literature and Reviews
4-(Trifluoromethyl)-2-pyridone (CAS number 50650-59-4), also known as 2-hydroxy-4-(trifluoromethyl)pyridine, bears the structure of 2-pyridone with an electron deficient trifluoromethyl group at 4-position of the pyridyl ring. 2-Pyridones are well-known organic compounds to form dimers via hydrogen bonding. 4-(Trifluoromethyl)-2-pyridone is a classic example that exists as tautomers, hence also known as 2-hydroxy-4-(trifluoromethyl)pyridine.
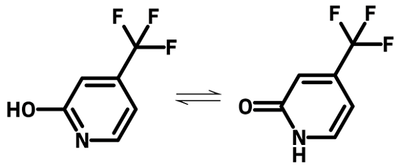
The trifluoromethyl group is considered to be one of the most powerful electron withdrawing groups yet with a significant electronegativity between that of fluorine and chlorine. Pyridone is a well known inhibitor for serotonin uptake used to treat major depressive disorder.
Multiple functional groups
For facile synthesis
Fluorinated pyridone building block
For semiconductors, photocatalysts, and semiconductors
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 50650-59-4 |
| Chemical Formula | C6H4F3NO |
| Full Name | 4-(Trifluoromethyl)-2-pyridone |
| Molecular Weight | 163.10 g/mol |
| Synonyms | 2-Hydroxy-4-(trifluoromethyl)pyridine, 4-(Trifluoromethyl)pyridin-2-ol |
| Classification / Family | Pyridone derivatives, Semiconductor synthesis intermediates, Organometallic compounds, Photocatalyst |
Chemical Structure
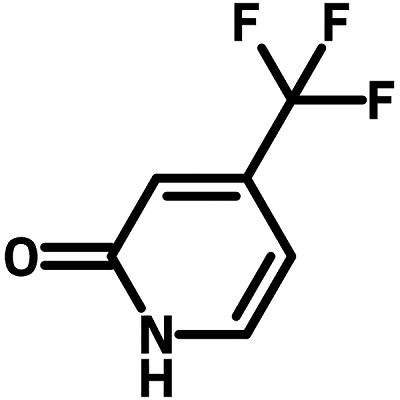
Product Details
| Purity | >98% (1H NMR) |
| Melting Point | Tm = 158 °C |
| Appearance | White powder |
MSDS Documentation
4-(Trifluoromethyl)-2-pyridone MSDS Sheet
Literature and Reviews
- Evaluation of chemical compounds for induction of male sterility in wheat (Triticum aestivum L.), K. Hakrabortyet al., Euphytica 147, 329–335 (2006); DOI: 10.1007/s10681-005-9025-z.
- Oxyfunctionalization of pyridine derivatives using whole cells of Burkholderia sp. MAK1, J. Stankevičiūtė et al., Sci. Rep. 6, 39129 (2016); DOI: 10.1038/srep39129.
- Synthesis of Novel Analogues of Marine Indole Alkaloids: Mono(indolyl)-4-trifluoromethylpyridines and Bis(indolyl)-4-trifluoromethylpyridines as Potential Anticancer Agents, W. Xiong et al., Bioorg. Med. Chem., 9 (7), 1773-1780 (2001); DOI: 10.1016/S0968-0896(01)00070-0.
