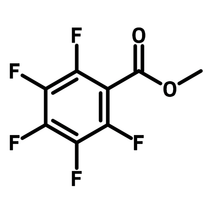
Methyl pentafluorobenzoate
CAS Number 36629-42-2
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, MonomersA perfluorinated benzoate building block
A synthesis intermediate for graft polymers, covalently bonded nanocomposites and luminescence metal complexes
Specifications | MSDS | Literature and Reviews
Methyl pentafluorobenzoate (CAS number 36629-42-2) or methyl perfluorobenzoate is a fully fluorinated benzoic acid methyl ester. Methyl pentafluorobenzoate is used to synthesize linkers for graft polymers such as perfluorophenyl azide with N-hydroxysuccinimide end group (PFPA-NHS). To synthesize PFPA-NHS, it begins with a nucleophilic aromatic substitution of sodium azide to the para-position of ester. The hydrolysis of the ester is followed forming an azide perfluorobenzoic acid. The last step involves an EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide) catalyzed coupling reaction of N-hydroxysuccinimide and the carboxylic acid. PFPA-NHS has been used for preparing polyaniline grafted graphene and catalase grafted polycaprolactone.
After the hydrolysis of the ester group, methyl pentafluorobenzoate binds to cadmium for luminescence metal complexes with overall quantum yield up to 39%.
Multiple functional groups
For facile synthesis
Fluorinated benzoate building block
For drug discovery, metal complexes and polymer chemistry
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
General Information
| CAS Number | 36629-42-2 |
| Chemical Formula | C8H3F5O2 |
| Full Name | Methyl pentafluorobenzoate |
| Molecular Weight | 226.10 g/mol |
| Synonyms | Pentafluorobenzoic acid methyl ester, Perfluorobenzoic acid methyl ester, Methyl perfluorobenzoate |
| Classification / Family | Fluorinated building blocks, Aromatic building blocks, Graft polymer, Luminescence metal complexes |
Chemical Structure
Product Details
| Purity | 97% |
| Boiling Point | Tb = 223 °C at 760 mmHg |
| Relative Density | 1.53 g/mL at 25 °C |
| Appearance | Colorless liquid |
MSDS Documentation
Methyl pentafluorobenzoate MSDS Sheet
Literature and Reviews
-
A simple route to functionalizing electrospun polymer scaffolds with surface biomolecules, K. Dziemidowicz et al., Int. J. Pharm., 597, 120231(2021); DOI: 10.1016/j.ijpharm.2021.120231.
-
Functionalization of pristine graphene for the synthesis of covalent graphene-polyaniline nanocomposite, J. Park et al., RSC Adv., 10, 26486(2020); DOI: 10.1039/d0ra03579c.
-
Photocatalytic C–F alkylation: facile access to multifluorinated arenes, A. Singh et al., Chem. Sci., 6, 7206(2015); DOI: 10.1039/c5sc03013g.
