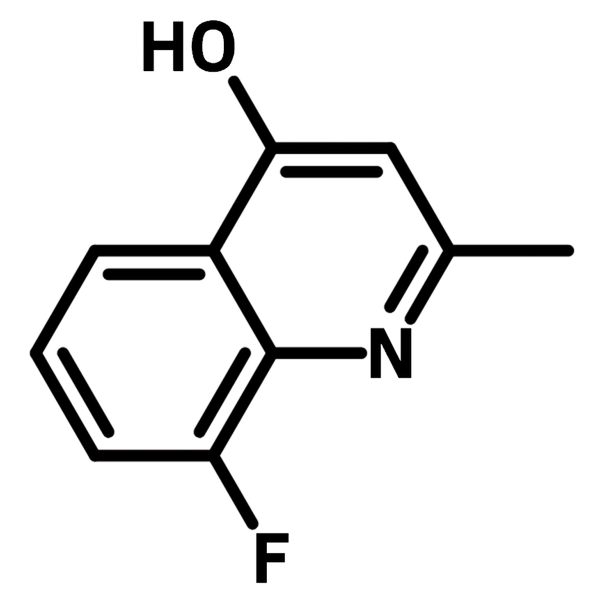
8-Fluoro-4-hydroxy-2-methylquinoline
CAS Number 5288-22-2
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials,A fluorinated heterocyclic building block
Materials synthesis in application of OLEDs, DSSCs and bioimaging
Specifications | MSDS | Literature and Reviews
8-Fluoro-4-hydroxy-2-methylquinoline (CAS number 5288-22-2) is consisting of a benzene-fused pyridine (quinoline) with 2-methyl, 4-hydroxyl and 8-fluoro substituents around the quinoline rings. 8-Fluoro-4-hydroxy-2-methylquinoline has multi-functional groups to allow facile reactions such as nucleophilic substitution and enamine condensation with an aldehyde. Quinoline is typically used to synthesize dyes for dye-sensitized solar cells (DSSCs) and OLEDs, due to its conjugated molecular structure. The energy gap of the molecule can be tuned effortlessly by functional group conversions.
The derivatives of quinoline are often bioactive, for instance, quinine is a well-known drug for malaria treatments. The active pharmaceutical ingredients can also be tailored into fluorescent bioimaging substances to monitor cell activities in vitro experiments.
Multiple functional groups
For facile synthesis
Worldwide shipping
Quick and reliable shipping
Fluorinated quinoline building block
For drug discoveries, OLEDs, and solar cells
High purity
>98% High purity
General Information
| CAS Number | 5288-22-2 |
| Chemical Formula | C10H8FNO |
| Full Name | 8-Fluoro-2-methyl-4-quinolinol |
| Molecular Weight | 177.18 g/mol |
| Synonyms | 8-Fluoro-2-methylquinolin-4-ol |
| Classification / Family | Quinoline derivatives, Heterocyclic building block, Fluorinated building block, Dyes, OLEDs, DSSCs |
Chemical Structure
Product Details
| Purity | >98% |
| Boiling Point | Tm = 230 °C – 236 °C |
| Appearance | Pale cream to pale brown powder/crystal |
MSDS Documentation
8-Fluoro-4-hydroxy-2-methylquinoline MSDS Sheet
Literature and Reviews
-
Application of quinoline derivatives in third-generation photovoltaics, G. Lewinska et al., J. Mater. Sci: Mater. Electron., 32, 18451-18456(2021); DOI: 10.1007/s10854-021-06225-6.
-
Influence of quinoline derivatives in I−/I3− redox electrolyte solution on the performance of Ru(II)-dye-sensitized nanocrystalline TiO2 solar cell, H. Kusama et al., J. Photochem. Photobiol. A: Chem., 165, 157-163(2004); DOI: 10.1016/j.jphotochem.2004.03.013.
-
Novel Ru(II) sensitizers bearing an unsymmetrical pyridine-quinoline hybrid ligand with extended π-conjugation: synthesis and application in dye-sensitized solar cells, G. Vougioukalakis et al., Dalton Trans., 42, 6582–6591(2013); DOI: 10.1039/c3dt33063j.
