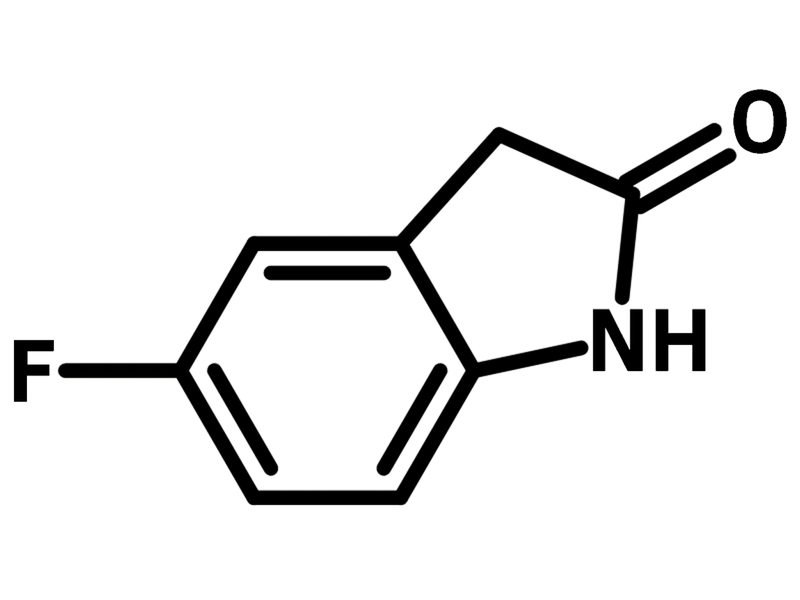
5-Fluorooxindole
CAS Number 56341-41-4
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials,A fluorinated heterocyclic building
Used as a synthesis intermediate for macromolecules and fluorescent dyes in application of APIs, bioimaging, solar cells and OLEDs
Specifications | MSDS | Literature and Reviews
5-Fluorooxindole (CAS number 56341-41-4), a fluorinated oxindole, is a benzene ring fused pyrrolidone (γ-lactam) with a fluorine substituent at 5-position. 5-Fluorooxindole has proven effectiveness on alleviating inflammatory pain and improving the analgesic effects of morphine. 5-Fluorooxindole inhibits the plasticity changes, oxidative stress, and inflammatory responses cause by peripheral inflammation. The derivatives of 5-fluorooxindole are used as α-glucosidase inhibitors for prevention and treatment of type 2 diabetes.
The 3-position of 5-fluorooxindole can be functionalized through an aldol condensation reaction in a basic solution. With the expanded chromophore, 5-fluorooxindole is used as an acceptor in dye-sensitized solar cells (DSSCs) with power conversion efficiency of 6.35%. Oxindole modified polymers are exploited as donors in non-fullerene polymer solar cells with Y6 (BTP-4F) as acceptor with power conversion efficiency up to 8.27%.
Multiple functional groups
For facile synthesis
Fluorinated oxindole building block
For drug discovery, solar cells, and OLEDs
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 56341-41-4 |
| Chemical Formula | C8H6FNO |
| Full Name | 5-Fluoro-2-oxindole |
| Molecular Weight | 151.14 g/mol |
| Synonyms | 5-Fluoro-1,3-dihydroindol-2-one, 5-Fluoro-2,3-dihydro-2-oxoindole |
| Classification / Family | Fluorinated building block, Heterocyclic building block, OLEDs, Solar cells |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 143 °C – 147 °C |
| Appearance | Off-white to pale pink/beige powder |
MSDS Documentation
Literature and Reviews
- Bis-arylidene oxindole-betulinic acid conjugate: a fluorescent cancer cell detector with potent anticancer activity, A. Pal et al, ACS Med. Chem. Lett., 6, 612−616(2015); DOI: 10.1021/acsmedchemlett.5b00095.
- New oxindole-bridged acceptors for organic sensitizers: substitution and performance studies in dye-sensitized solar cells, Y. Tingare et al., Molecules, 25, 2159(2020); DOI: 10.3390/molecules25092159.
- Synthesis and biological evaluation of 5-fluoro-2-oxindole derivatives as potential α-glucosidase inhibitors, J. Lin et al., Front. Chem., 10, 928295(2022); DOI: 10.3389/fchem.2022.928295.
