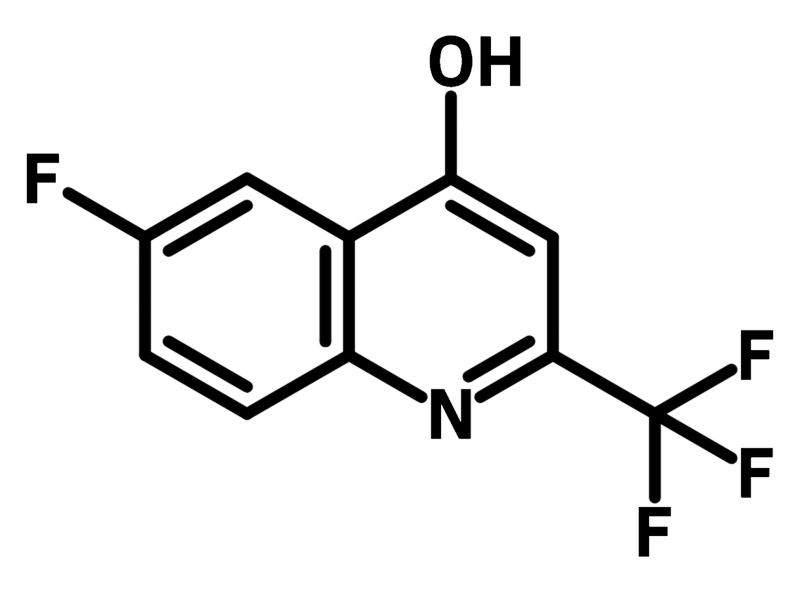
6-Fluoro-4-hydroxy-2-(trifluoromethyl)quinoline
CAS Number 31009-34-4
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials, MonomersA fluorinated hydroxyquinoline building block
Used as an intermediate for the synthesis of antitubercular and antiplasmodial agents
Specifications | MSDS | Literature and Reviews
6-Fluoro-4-hydroxy-2-(trifluoromethyl)quinoline (CAS number 31009-34-4) is a derivative of quinoline, substituted with trifluoromethyl, hydroxyl and fluoride groups. 6-Fluoro-4-hydroxy-2-(trifluoromethyl)quinoline can exist in a tautomeric form, known as quinolone, where the hydroxy group becomes a ketone and the imine changes to an amine. 6-Fluoro-4-hydroxy-2-(trifluoromethyl)quinoline serves as a precursor for synthesizing thioquinolines, which are used to develop non-cytotoxic, potent and selective antitubercular agents. The thiolation reaction is carried out using phosphorus pentasulfide in pyridine.
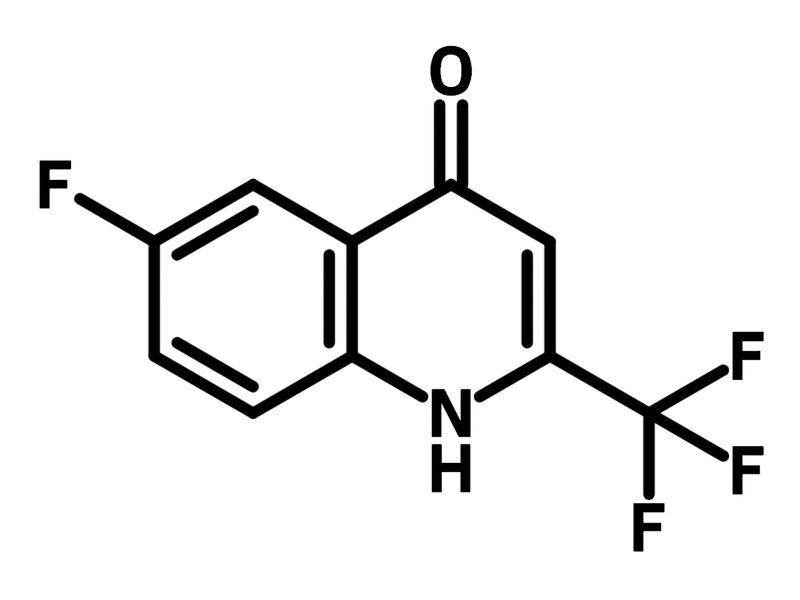
Furthermore, the hydroxyl group in 6-fluoro-4-hydroxy-2-(trifluoromethyl)quinoline can undergo nucleophilic substitution to synthesis antiplasmodial agents for the treatment of malaria.
Multiple functional groups
For facile synthesis
Fluorinated quinoline building block
For drug discovery, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 31009-34-4 |
| Chemical Formula | C10H5F4NO |
| Full Name | 6-Fluoro-2-(trifluoromethyl)-4-quinolinol |
| Molecular Weight | 231.15 g/mol |
| Synonyms | 6-fluoro-2-(trifluoromethyl)quinolin-4-ol |
| Classification / Family | Fluorinated building blocks, Heterocyclic building blocks, Quinoline building blocks, APIs |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 259 °C – 263 °C |
| Appearance | Off-white powder |
MSDS Documentation
6-Fluoro-4-hydroxy-2-(trifluoromethyl)quinoline MSDS Sheet
Literature and Reviews
- Design of non-nucleoside inhibitors of HIV-1 reverse transcriptase with improved drug resistance properties, G. Freeman et al., J. Med. Chem., 47(24), 5923–5936(2004); DOI: 10.1021/jm040072r.
- Measurement of interfluorine distance in solids, M. Gilchrist et al., J. Magn. Reson., 152, 1–6(2001), DOI: 10.1006/jmre.2001.2351.
- 4-Substituted thioquinolines and thiazoloquinolines: potent, selective, and Tween-80 in vitro dependent families of antitubercular agents with moderate in vivo activity, ChemMedChem.,6(12), 2252–2263(2011); DOI: 10.1002/cmdc.201100309.
