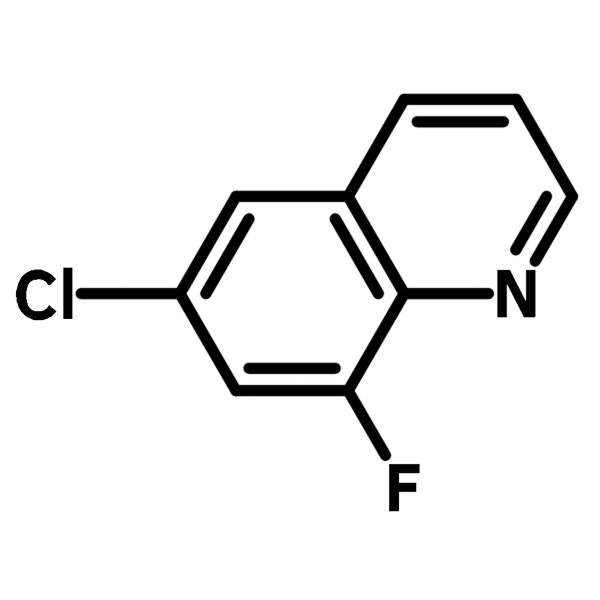
6-Chloro-8-fluoroquinoline
CAS Number 52200-53-0
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials,A fluorinated heterocyclic building block
Used as a synthesis intermediate for APIs, DSSCs and sensors
Specifications | MSDS | Literature and Reviews
6-Chloro-8-fluoroquinoline (CAS number 52200-53-0) is a fluorinated quinoline derivative with a chlorine and a fluorine at 6- and 8-position respectively. This building block is best known for its used in active pharmaceutical ingredients (APIs), such as Besifloxacin and Clinafloxacin in topical antibiotic treatments and conjunctive bacterial infections. Other than pharmaceutical uses, 6-Chloro-8-fluoroquinoline is a synthesis intermediate for Schiff bases, through nucleophilic aromatic substitution. These Schiff bases are used for metal-ion recognition sensors because their fluorescent emissions shift after chelating to a metal center.
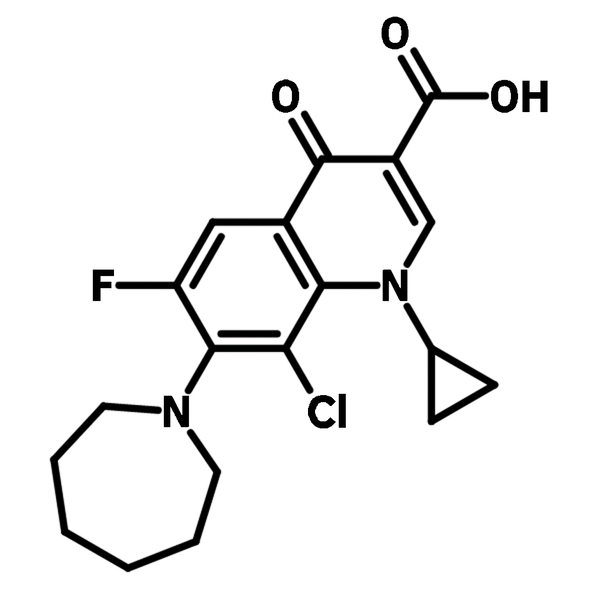
The Schiff bases formed by this building block are typically bidentate/tridentate ligands which readily coordinate to Ru and Ir ion centers to form dye complexes in applications of dye-sensitized solar cells(DSSCs).
Multiple functional groups
For facile synthesis
Worldwide shipping
Quick and reliable shipping
Fluorinated quinoline building block
For drug discoveries, dyes, and solar cells
High purity
>98% High purity
General Information
| CAS Number | 52200-53-0 |
| Chemical Formula | C9H5ClFN |
| Full Name | 6-Chloro-8-fluoroquinoline |
| Molecular Weight | 181.59 g/mol |
| Synonyms | N/A |
| Classification / Family | Fluorinated building block, Heterocyclic building block, Quinoline derivatives, Schiff base, Dyes, Semiconductor synthesis intermediates, organic photovoltaics |
Chemical Structure
Product Details
| Purity | >98% |
| Melting Point | Tm = 79 °C – 81 °C |
| Appearance | White to yellow crystal |
MSDS Documentation
6-Chloro-8-fluoroquinoline MSDS Sheet
Literature and Reviews
-
Besifloxacin ophthalmic suspension 0.6% in the treatment of bacterial conjunctivitis patients with Pseudomonas aeruginosa infections, B. Silverstein et al., Clin. Ophthalmol., 6, 1987-1996(2012); DOI: 10.2147/OPTH.S35715.
-
Clinafloxacin for the treatment of bacterial endocarditis, D. Levine et al., Clin. Infect. Dis., 38(5), 620-631(2004); DOI: 10.1086/381670.
-
Copper(II) complexes based on quinoline-derived Schiff-base ligands: synthesis, characterization, HSA/DNA binding ability, and anticancer activity, Med. Chem. Commun., 9, 1663–1672(2018); DOI: 10.1039/c8md00223a.
