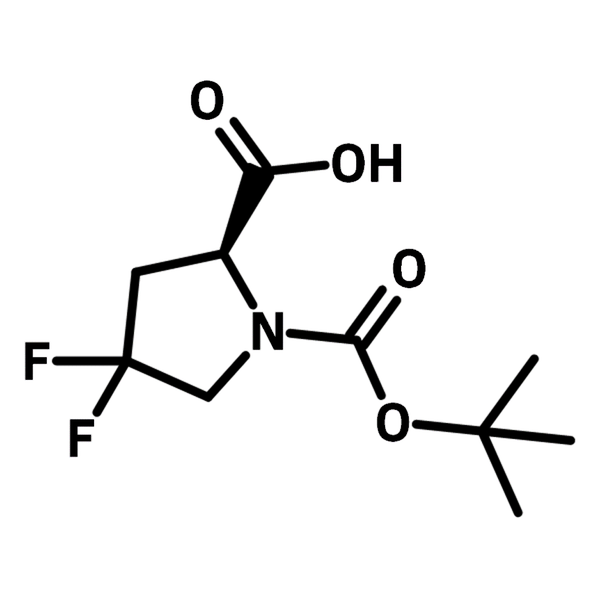
N-Boc-4,4-difluoro-L-proline
CAS Number 203866-15-3
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials,A difluorinated l-proline derivative
Used as a molecular scaffold for biochemistry and a conformational tool for polypeptides
Specifications | MSDS | Literature and Reviews
N-Boc-4,4-difluoro-l-proline (CAS number 203866-15-3) is a tert-butoxycarbonyl (Boc) protected l-proline with two fluorine substituents at 4-position. N-Boc-4,4-difluoro-l-proline is an S-enantiomer, making it an excellent additive/catalyst in asymmetric reactions. In protein/peptide chemistry, N-Boc-4,4-difluoro-l-proline is introduced for conformational control favoring 78% cis-conformers. The conformation selectivity is also expressed in the 19F NMR spectrum which is a powerful tool to study the isomerization of peptides and proteins.
N-Boc-4,4-difluoro-l-proline is also applied as a molecular scaffold for synthesizing fibroblast activation protein, a trans-membrane serine protease.
Multiple functional groups
For facile synthesis
Fluorinated l-proline building block
For organic synthesis, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
General Information
| CAS Number | 203866-15-3 |
| Chemical Formula | C10H15F2NO4 |
| Full Name | (S)-1-(tert-Butoxycarbonyl)-4,4-difluoro-2-pyrrolidinecarboxylic acid |
| Molecular Weight | 251.23 g/mol |
| Synonyms | N-(tert-Butoxycarbonyl)-4,4-difluoro-l-proline |
| Classification / Family | Fluorinated building blocks, Heterocyclic building blocks, Proteins, Peptides, Asymmetric synthesis, Chiral molecules |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | Tm = 120 °C – 140 °C |
| Optical Rotation | −44.19 ° (c = 1, methanol) |
| Appearance | Off-white powder |
MSDS Documentation
N-Boc-4,4-difluoro-l-proline MSDS Sheet
Literature and Reviews
- Deciphering the fluorine code—the many hats fluorine wears in a protein environment, A. Berger et al., Acc. Chem. Res., 2017, 50, 2093–2103(2017); DOI: 10.1021/acs.accounts.7b00226.
- Minimizing conformational bias in fluoroprolines through vicinal difluorination, G.-J. Hofman et al., Chem. Commun., 54, 5118(2018); DOI: 10.1039/c8cc01493k.
- Fluorinated prolines as conformational tools and reporters for peptide and protein chemistry, S. Verhoork et al., Biochemistry, 57(43), 6132–6143(2018); DOI: 10.1021/acs.biochem.8b00787.
