4-Fluoro-3-methylbenzoic acid
CAS Number 403-15-6
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers,A fluorinated benzoic acid building block
For the synthesis of APIs
Specifications | MSDS | Literature and Reviews
4-Fluoro-3-methylbenzoic acid (CAS number 403-15-6), is a meta-toluic acid with a fluoride group at the 4-position. 4-Fluoro-3-methylbenzoic acid serves as a versatile building block for the synthesis of active pharmaceutical ingredients (APIs), as its functional group can be directly used or easily converted to alternative functional groups. The carboxylic acid group of 4-fluoro-3-methylbenzoic acid enables it to be attached to molecular scaffolds. Alternatively, it can be reduced to a hydroxy/aldehyde group for other type of reactions. The methyl group can be functionalized by bromide through a benzylic bromination reaction. The resulting brominated product can then be applied in the synthesis of bicyclic heterocycles.
An analog of imidazopyridine based on 4-fluoro-3-methylbenzoic acid exhibits a potency of 0.1 nM as an anticoccidial agent.
Multiple functional groups
For facile synthesis
Fluorinated benzoic acid building block
For drug discovery, medicinal chemistry, and biochemistry
Low Cost
Competitively priced, high quality product
High purity
>97% High purity
General Information
| CAS Number | 403-15-6 |
| Chemical Formula | C8H7FO2 |
| Full Name | 4-Fluoro-3-methylbenzoic acid |
| Molecular Weight | 154.14 g/mol |
| Synonyms | 4-Fluoro-m-toluic acid |
| Classification / Family | Fluorinated building block, Benzoic acid building blocks, APIs |
Chemical Structure
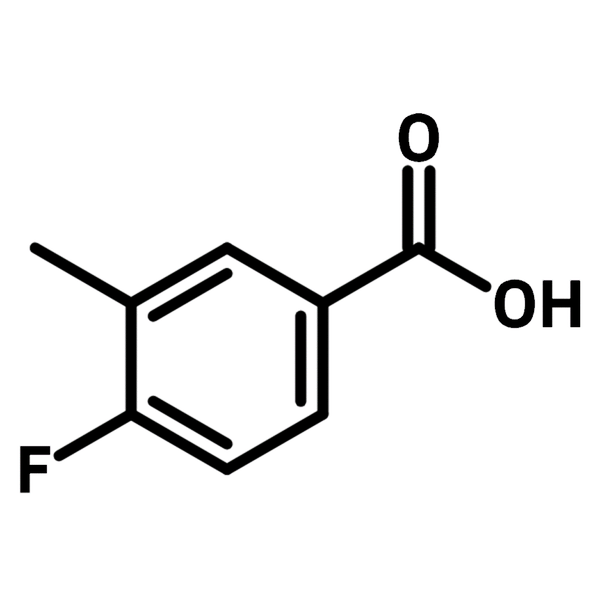
Product Details
| Purity | 97% |
| Melting Point | Tm = 164 °C – 168 °C |
| Appearance | White powder |
MSDS Documentation
4-Fluoro-3-methylbenzoic acid MSDS Sheet
Literature and Reviews
- Improved loading and cleavage methods for solid-phase synthesis using chlorotrityl resins: synthesis and testing of a library of 144 discrete chemicals and potential farnesyltransferase inhibitors, J Park et al., J. Comb. Chem., 6 (3), 407–413 (2004); DOI: 10.1021/cc0340729.
- Enantiospecific synthesis of SB 214857, a potent, orally active nonpeptide fibrinogen receptor antagonist, W. Miller et al., Tetrahedron Lett., 36 (52), 9433–9436 (1995); DOI: 10.1016/0040-4039(95)02054-3.
- Structure-based design, synthesis and biological evaluation of highly selective and potent G protein-coupled receptor kinase 2 inhibitors, H. Waldschmidt et al., J. Med. Chem., 59 (8), 3793–3807 (2016); DOI: 10.1021/acs.jmedchem.5b02000.
