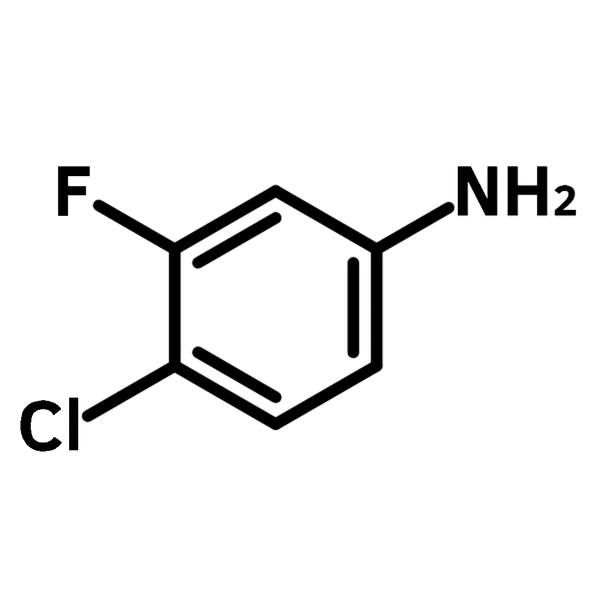
4-Chloro-3-fluoroaniline
CAS Number 367-22-6
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, MonomersA dihalogenated aniline building block
Used as a building block for introducing a halogenated aniline moiety to molecules in medicinal chemistry and as a monomer for semiconducting polyanilines
Specifications | MSDS | Literature and Reviews
4-Chloro-3-fluoroaniline (CAS number 367-22-6) is an aniline derivative featuring chloride and fluoride substituents at para- and meta-positions. 4-Chloro-3-fluoroaniline serves as a building block in the synthesis of antimalarial agents, wherein it undergoes nucleophilic substitution reactions. The incorporation of chloride and fluoride substituents significantly improves the drug’s potency, reducing the half maximal effective concentration (EC50) down to 27 nM against Plasmodium falciparum parasite. The utility of 4-chloro-3-fluoroaniline building block can be found in many active pharmaceutical ingredients (APIs) such as piperidine derivatives for antiviral treatments and quinazolines as anticancer agents.
Additionally, 4-chloro-3-fluoroaniline is also an optimal monomer for synthesizing fluorinated polyanilines, via Buchwald-Hartwig amination.
Multiple functional groups
For facile synthesis
Fluorinated aniline building block
For semiconductors, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 367-22-6 |
| Chemical Formula | C6H5ClFN |
| Full Name | 4-Chloro-3-fluoroaniline |
| Molecular Weight | 145.56 g/mol |
| Synonyms | 4-Chloro-3-fluorophenylamine, 4-chloro-3-fluorobenzenamine |
| Classification / Family | Fluorinated building blocks, Aniline building blocks, APIs, Semiconductors |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 58 °C – 62 °C |
| Appearance | Grey powder/crystals |
MSDS Documentation
4-Chloro-3-fluoroaniline MSDS Sheet
Literature and Reviews
- A short review on synthetic strategies towards quinazoline based anticancer drugs, V. Sharma et al., Arkivoc, IX, 150–176(2021); DOI: 10.24820/ark.5550190.p011.552.
- Optimization of 2-anilino 4-amino substituted quinazolines into potent antimalarial agents with oral in vivo activity, P. Gilson et al., J. Med. Chem., 60, 1171–1188(2017); DOI: 10.1021/acs.jmedchem.6b01673.
- Imidazolopiperazines: hit to lead optimization of new antimalarial agents, T. Wu et al., J. Med. Chem., 54, 5116–5130(2011); DOI: 10.1021/jm2003359.
