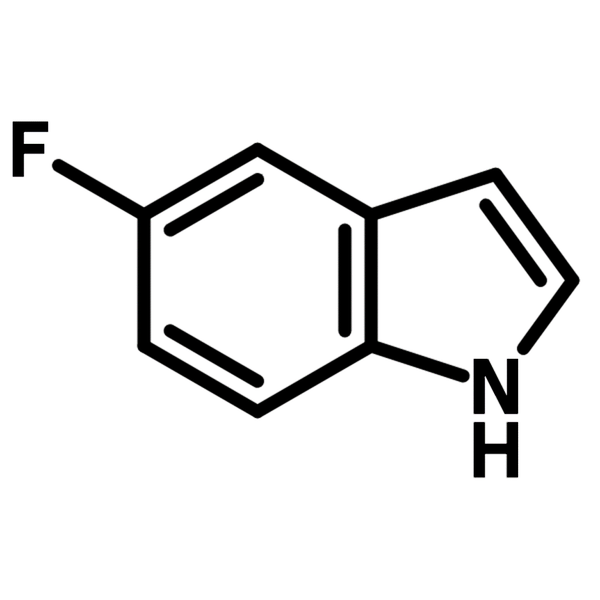
5-Fluoroindole
CAS Number 399-52-0
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials,A fluorinated indole
Used as a synthesis intermediate for APIs and polyindoles in application of biosynthesis, OLEDs, DSSCs and semiconductors
Specifications | MSDS | Literature and Reviews
5-Fluoroindole (CAS number 399-52-0), derived from indole, a bicyclic heterocycle, has a fluorine substituent at 5-position. 5-Fluoroindole is applied as a fluorine labeled indole in tryptophan biosynthesis which can be monitored by 19F NMR. The conversion of 5-fluoroindole to 5-fluorotryphtophan is catalyzed by an enzyme synthase with L-serine in proliferating Escherichia coli cells.
5-Fluoroindole is also used to synthesize polyindoles. The oxidative potential of 5-fluoroindole is 1.05 V vs SCE (saturated calomel electrode), which allows it to be easily polymerized electrochemically. The fluorinated polyindoles show conductivity of 7.1 × 10-2 S/cm as well as redox properties. Polymers of 5-fluoroindole also exhibit blue light emission in fluorescent spectroscopy, suggesting their potential applications in OLEDs.
Multiple functional groups
For facile synthesis
Fluorinated indole building block
For drug discovery, OLEDs, and semiconductors
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 399-52-0 |
| Chemical Formula | C8H6FN |
| Full Name | 5-Fluoro-1H-indole |
| Molecular Weight | 135.14 g/mol |
| Synonyms | N/A |
| Classification / Family | Fluorinated building block, Heterocyclic building block, APIs, OLEDs, DSSCs, Semiconductors |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 45 °C – 48 °C |
| Appearance | Off-white to pale beige powder |
MSDS Documentation
Literature and Reviews
-
Low-potential electrochemical polymerization of 5-fluoroindole and characterization of its polymers, G. Nie et al., J. Electroanal. Chem., 604(2), 125−132(2007); DOI: 10.1016/j.jelechem.2007.03.010.
-
Assessing the applicability of 19F labeled tryptophan residues to quantify protein dynamics, C. Krempl et al., J. Biomol. NMR, 77, 55−67(2023); DOI: 10.1007/s10858-022-00411-2.
-
Modulation of the La/Lb mixing in an indole derivative: a position-dependent study using 4-, 5-, and 6-fluoroindole, J. Wilke et al., J. Phys. Chem. A, 121, 1597−1606(2017); DOI: 10.1021/acs.jpca.6b12605.
