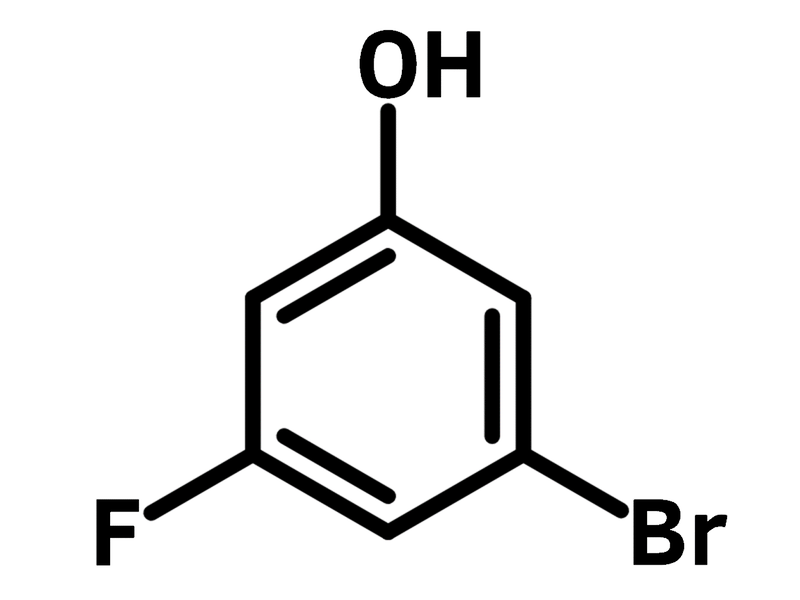
3-Bromo-5-fluorophenol
CAS Number 433939-27-6
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, MonomersA fluorinated phenol building block
As a tri-functionalized molecular scaffold for the synthesis of APIs
Specifications | MSDS | Literature and Reviews
3-Bromo-5-fluorophenol (CAS number: 433939-27-6), is a phenol derivative that contains both bromide and fluoride functional groups. The three functional groups of 3-bromo-5-fluorophenol exhibit distinct reactivity. The hydroxy group is capable of undergoing nucleophilic substitution and Mitsunobu reactions, while the bromo-group is well-suited for metal-catalyzed cross-coupling reactions. The fluoride substituent reacts with nucleophiles via nucleophilic aromatic substitution, or it can be preserved to enhance the binding affinity of synthesized active pharmaceutical ingredients (APIs).
3-Bromo-5-fluorophenol is commonly utilized as a molecular scaffold for APIs. Anticancer 3,4'-substituted diaryl guanidinium derivatives are synthesized from 3-bromo-5-fluorophenol, demonstrating an inhibition of 4.07 ± 0.10 µM against the human promyelocytic leukaemia (HL-60) cell line.
Multiple functional groups
For facile synthesis
Fluorinated phenol building block
For drug discovery, medicinal chemistry, and organic synthesis
Low Cost
Competitively priced, high quality product
High purity
>98% High purity
General Information
| CAS Number | 433939-27-6 |
| Chemical Formula | C6H4BrFO |
| Full Name | 3-Bromo-5-fluorophenol |
| Molecular Weight | 191.00 g/mol |
| Synonyms | 3-Fluoro-5-Bromophenol |
| Classification / Family | Fluorinated building block, Halogenated building blocks, Phenol building blocks, APIs |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 36 °C – 40 °C |
| Appearance | White Powder |
MSDS Documentation
3-Bromo-5-fluorophenol MSDS Sheet
Literature and Reviews
- Mosquito acetylcholinesterase as a target for novel phenyl-substituted carbamates, J. Mutunga et al., Int. J. Environ. Res. Public Health, 16, 1500 (2019); DOI: 10.3390/ijerph16091500.
- The twin drug approach for novel nicotinic acetylcholine receptor ligands, Bioorg. Med. Chem., 23 (15), 4375–4389 (2015); DOI: 10.1016/j.bmc.2015.06.034.
- Exploring the anti-cancer mechanism of novel 3,4'-substituted diaryl guanidinium derivatives, V. Previtali et al., Pharmaceuticals, 13, 485 (2020); DOI: 10.3390/ph13120485.
