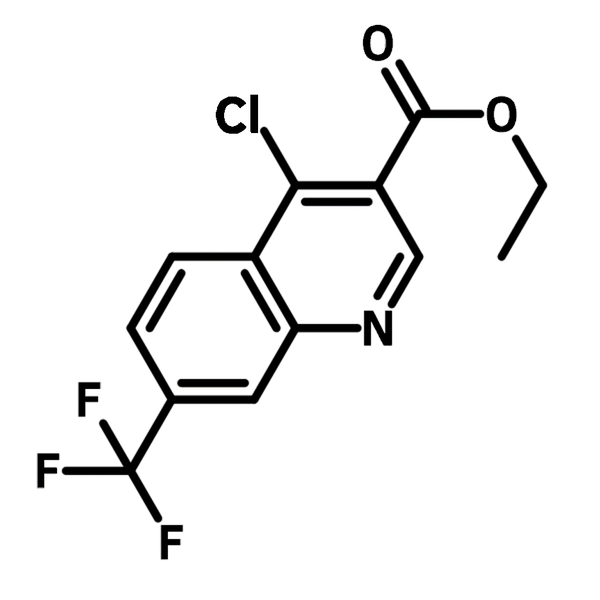
4-Chloro-7-trifluoromethylquinoline-3-carboxylic acid ethyl ester
CAS Number 1168-42-3
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials,A fluorinated heterocyclic building block
As an intermediate for the synthesis of APIs and DSSCs
Specifications | MSDS | Literature and Reviews
4-Chloro-7-trifluoromethylquinoline-3-carboxylic acid ethyl ester (CAS number 1168-42-3) is an ethyl ester of quinoline-3-carboxylic acid with both electron-withdrawing chloro and trifluoromethyl groups. Like other quinoline derivatives, 4-chloro-7-trifluoromethylquinoline-3-carboxylic acid ethyl ester is used as antimicrobials in medicinal chemistry research. Study suggests that attaching this building block to a cell-penetrating peptides causes the osmotic swelling of endosome, thus resulting an enhancement of cell penetration ability of the peptides. During the cell penetrating process, trifluoromethyl group binds with protons, followed by the increase of pH and destabilization of the cell membranes.
The lone pair on the amine can bind to metal centers forming dye complexes in application of dye-sensitized solar cells (DSSCs). The chloro substituent enables the further functionalization to the molecule such as forming a bidentate ligand for bioimaging and sensors.
Multiple functional groups
For facile synthesis
Fluorinated quinoline building block
For drug discoveries, dyes, and solar cells
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 21168-42-3 |
| Chemical Formula | C13H9ClF3NO2 |
| Full Name | Ethyl 4-chloro-7-(trifluoromethyl)-3-quinolinecarboxylate |
| Molecular Weight | 303.66 g/mol |
| Synonyms | N/A |
| Classification / Family | Quinoline derivatives, Fluorinated building blocks, Heterocyclic building block, Dyes, DSSCs, APIs |
Chemical Structure
Product Details
| Purity | >98% |
| Melting Point | Tm = 71 °C – 72 °C |
| Appearance | Solid |
MSDS Documentation
4-Chloro-7-trifluoromethylquinoline-3-carboxylic acid ethyl ester MSDS Sheet
Literature and Reviews
-
An extensive review on biological interest of quinoline and its analogs, V. Snehi et al., Int. J. Health Sci. Res., 8(1), 45-66(2023); DOI: 10.52403/ijshr.20230105.
-
Antimicrobial peptides and cell-penetrating peptides: non-antibiotic membrane-targeting strategies against bacterial infections, X. Huang et al., Infect. Drug Resist., 16, 1203-1219(2023); DOI: 10.2147/IDR.S396566.
-
Influence of quinoline derivatives in I−/I3− redox electrolyte solution on the performance of Ru(II)-dye-sensitized nanocrystalline TiO2 solar cell, H. Kusama et al., J. Photochem. Photobiol. A, 165, 157-163(2004); DOI: 10.1016/j.jphotochem.2004.03.013.
