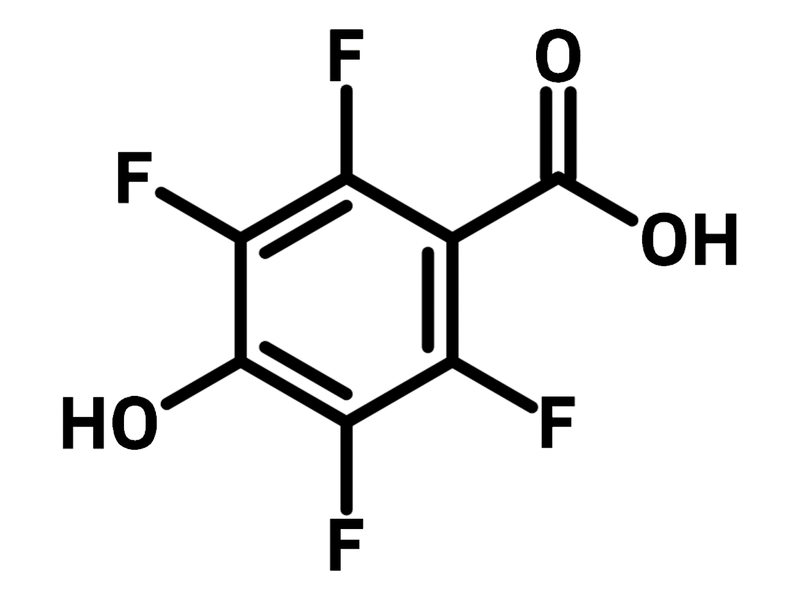
2,3,5,6-Tetrafluoro-4-hydroxybenzoic acid
CAS Number 652-34-6
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, MonomersA perfluorinated hydroxybenzoic acid building block
Used as a synthesis intermediate for APIs and quenched activity-based probe for bioimaging applications.
Specifications | MSDS | Literature and Reviews
2,3,5,6-Tetrafluoro-4-hydroxybenzoic acid (CAS number 652-34-6) is a perfluorinated para-hydroxybenzoic acid building block. Due to its perfluorination, 2,3,5,6-tetrafluoro-4-hydroxybenzoic acid is relatively acidic with an observed pKa of 5.3, compared to non-fluorinated 4-hydroxybenzoic acid (pKa of 9.3). 2,3,5,6-Tetrafluoro-4-hydroxybenzoic acid is a precursor used to synthesize BMV109 which serves as a fluorescent activity-based probe for bioimaging. This probe is intrinsically non-fluorescent but only becomes fluorescent after reacting with a target protease.
2,3,5,6-Tetrafluoro-4-hydroxybenzoic acid is also employed in the preparation of novel inhibitors for protein farnesyltransferase (FTase) and geranylgeranyltransferase (GGTase), through Mitsunobu reaction. Enzyme inhibition studies demonstrate a potency of 2.9 µM against FTase and 7.5 µM against GGTase.
Multiple functional groups
For facile synthesis
Fluorinated hydroxybenzoic acid building block
For drug discovery, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 652-34-6 |
| Chemical Formula | C7H2F4O3 |
| Full Name | 2,3,5,6-Tetrafluoro-4-hydroxybenzoic acid |
| Molecular Weight | 210.08 g/mol |
| Synonyms | 4-Carboxy-2,3,5,6-tetrafluorophenol, 4-Hydroxy-2,3,5,6-tetrafluorobenzoic acid |
| Classification / Family | Fluorinated building blocks, Benzoic acid building blocks, APIs, Bioimaging |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 151 °C – 153 °C |
| Appearance | White powder |
MSDS Documentation
2,3,5,6-Tetrafluoro-4-hydroxybenzoic acid MSDS Sheet
Literature and Reviews
- Live cell imaging and profiling of cysteine cathepsin activity using a quenched activity-based probe, L. Edgington-Mitchell et al., Methods Mol. Biol., 1491, 145-159(2017); DOI: 10.1007/978-1-4939-6439-0_11.
- An improved quenched fluorescent probe for imaging of cysteine cathepsin activity, M. Verdoes et al., J. Am. Chem. Soc., 135(39), 14726–14730(2013); DOI: 10.1021/ja4056068.
- Synthesis of the farnesyl ether 2,3,5-trifluoro-6-hydroxy-4-[(E,E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yloxy]nitrobenzene, and related compounds containing a substituted hydroxytrifluorophenyl residue: novel inhibitors of protein farnesyltransferase, geranylgeranyltransferase I and squalene synthase, J. Marriott et al., J. Chem. Soc., Perkin Trans., 1, 4265–4278(2000); DOI: 10.1039/b007101n.
