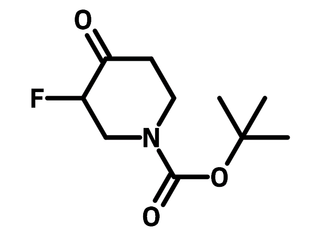
1-(tert-Butoxycarbonyl)-3-fluoro-4-piperidone
CAS Number 211108-50-8
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, MonomersA fluorinated piperidone building block
As a molecular scaffold for drug discovery and bioimaging
Specifications | MSDS | Literature and Reviews
1-(tert-Butoxycarbonyl)-3-fluoro-4-piperidone (CAS number 211108-50-8), also known as 1-Boc-3-fluoro-4-piperidone, is a Boc (tert-butoxycarbonyl) protected piperidone bearing a fluorine substituent on 3-position. 1-(tert-Butoxycarbonyl)-3-fluoro-4-piperidone is the core of fentanyl, an opioid drug used as an analgesic. Fentanyl binds to opioid receptors leading to the inhibitory effects. To synthesize fentanyl derivatives, 1-(tert-butoxycarbonyl)-3-fluoro-4-piperidone reacts with aniline via reductive amination followed by nucleophilic substitution of propionyl chloride. Boc group is then removed with trifluoroacetic acid (TFA) and phenylethyl group is attached to form fentanyl.
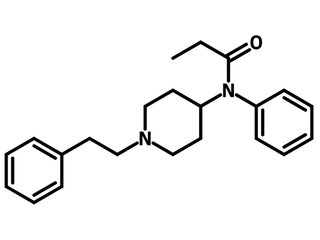
Photochromic functional groups (such as azobenzene) can be easily attached to 1-(tert-butoxycarbonyl)-3-fluoro-4-piperidone for optically controlled μ-opioid receptor activation. The trans- and cis- formations of azo result significantly different receptor affinities that are photoswitchable. 1-(tert-Butoxycarbonyl)-3-fluoro-4-piperidone functionalized with chromophores can also be applied for bioimaging to study the binding affinity of the molecules.
Multiple functional groups
For facile synthesis
Fluorinated piperidone building block
For drug discovery, medicinal chemistry, and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
General Information
| CAS Number | 211108-50-8 |
| Chemical Formula | C10H16FNO3 |
| Full Name | 1-(tert-Butoxycarbonyl)-3-fluoro-4-piperidone |
| Molecular Weight | 217.24 g/mol |
| Synonyms | 1-Boc-3-fluoro-4-piperidone, tert-Butyl 3-fluoro-4-oxo-1-piperidinecarboxylate, 3-Fluoro-4-oxo-1-piperidinecarboxylic acid tert-butyl ester, 1-Boc-3-fluoro-4-oxopiperidine |
| Classification / Family | Fluorinated building block, Heterocyclic building block, Drug discovery, Bioimaging |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | Tm = 74.0 – 78.0 °C |
| Appearance | White to off-white powder |
MSDS Documentation
1-(tert-Butoxycarbonyl)-3-fluoro-4-piperidone MSDS Sheet
Literature and Reviews
-
Photochromic fentanyl derivatives for controlled μ-opioid receptor activation, R. Lahmy et al., Chem. Eur. J., 28, e202201515(2022); DOI: 10.1002/chem.202201515.
-
Rapid and efficient microwave-assisted Friedländer quinoline synthesis, H. Bailey et al., ChemistryOpen, 9, 1113–1122(2020); DOI: 10.1002/open.202000247.
-
Evaluation of the alicyclic Gauche effect in 2-Fluorocyclohexanone analoges: a combined NMR and DFT study, D. Silva et al., Eur. J. Org. Chem., 884–890(2020); DOI: 10.1002/ejoc.201901815.
