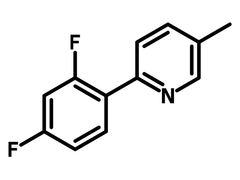
2-(2,4-Difluorophenyl)-5-methylpyridine
CAS Number 583052-21-5
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials,A difluorinated heterocyclic building block
As cyclometalating ligand to form coordinative complexes in application of OLEDs, DSSCs and photocatalysis reactions
Specifications | MSDS | Literature and Reviews
2-(2,4-Difluorophenyl)-5-methylpyridine (DFPMPy), CAS number 583052-21-5, is a difluorinated heterocyclic derivative of arylpyridine with enhanced solubility by the methyl group. DFPMPy is often used as a cyclometalating ligand with the nitrogen electron lone pair and the delocalized π-electron cloud in the benzene ring being coordinated to a metal center. The metal-ligand complexes have demonstrated excellent photocatalytic capability in visible-light photoredox reactions. During the photoredox process, an electron migrates from the metal center to the ligands, also known as metal-ligand charge transfer (MLCT) after irradiation as the complex reaches the excited state. After the single-electron transfer, the excited complex regains an electron from another reagent and restart the photoredox catalytic cycle.
These complexes are also synthesized for light-emitting electrochemical cells with photoluminescent quantum yield up to 93%. The fluorine-substituents lower the highest occupied molecular orbital (HOMO) energy level resulting shifts in the emission spectrum. Arylpyridine-iridium complexes are widely investigated in water splitting to generate hydrogen. As photosensitizers, these complexes are also used in dye-sensitized solar cells (DSSCs).
Multiple functional groups
For facile synthesis
Fluorinated phenylpyridine building block
For drug discoveries, solar cells, and photocatalists
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
General Information
| CAS Number | 583052-21-5 |
| Chemical Formula | C12H9F2N |
| Full Name | 2-(2,4-Difluorophenyl)-5-methylpyridine |
| Molecular Weight | 205.21 g/mol |
| Synonyms | DFPMPy |
| Classification / Family | Pyridine derivatives, Fluorinated building blocks, Heterocyclic building block, Dyes, DSSCs, Photocatalyst ligands, OLEDs |
Chemical Structure
Product Details
| Purity | >97% |
| Melting Point | Tm = 55 °C |
| Appearance | White/off-white to yellow to light brown powder/crystal |
MSDS Documentation
2-(2,4-Difluorophenyl)-5-methylpyridine MSDS Sheet
Literature and Reviews
-
Visible light driven generation and alkyne insertion reactions of stable bis-cyclometalated Pt(IV) hydrides, D. Poveda et al., Chem. Sci., 11, 12095–12102(2020); DOI: 10.1039/d0sc04879h.
-
Cationic iridium (III) complexes bearing ancillary 2,5-dipyridyl(pyrazine) (2,5-dpp) and 2,2’:5’,2’’- terpyridine (2,5-tpy) ligands: synthesis, optoelectronic characterization and light-emitting electrochemical cells, K. Hasan et al.,
Dalton Trans., 43, 13672-13682(2014); DOI: 10.1039/C4DT02100B.
-
Generation of phosphoranyl radicals via photoredox catalysis enables voltage−independent activation of strong C−O bonds, E. Stache et al., ACS Catal., 8(12), 11134–11139(2018); DOI: 10.1021/acscatal.8b03592.
