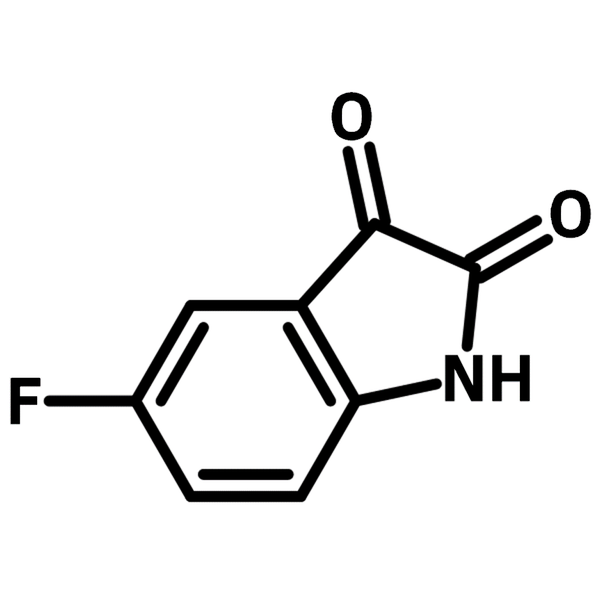
5-Fluoroisatin
CAS Number 443-69-6
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials, MonomersA fluorinated heterocyclic building block
Used as a synthesis intermediate for Schiff bases, macromolecules, and metal complexes in application of catalysts, OLEDs, and APIs
Specifications | MSDS | Literature and Reviews
5-Fluoroisatin (CAS number 443-69-6), a fluorinated indole derivative, contains a fluorine (5-position) and two carbonyls (2,3-positions). The carbonyl group at 3-position reacts with amines or hydrazines for imines or hydrazones that function as Schiff bases. The Schiff base based on 5-fluoroisatin coordinates to cobalt, yielding Co-isatin complexes. The metal complexes catalyze oxidative condensation reaction in air by generating singlet oxygen under sunlight. A macromolecule with donor-acceptor-donor system can be synthesized with 5-fluoroisatin and a diamine building block. The OLED device based on the macromolecule shows external quantum efficiency of 0.0515% with emission of 630 to 700 nm.
The 5-membered ring of 5-fluoroisatin can be expanded, forming isatoic anhydride through Baeyer-Villiger oxidation with mCPBA (meta-chloroperoxybenzoic acid). 5-Fluoroisatin can also be reshaped to a quinoline under Pfitzinger reaction, providing a platform for post-modification of the isatin derivatives.
Multiple functional groups
For facile synthesis
Fluorinated isatin building block
For drug discovery, catalysts, and OLEDs
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 443-69-6 |
| Chemical Formula | C8H4FNO2 |
| Full Name | 5-Fluoro-1H-indole-2,3-dione |
| Molecular Weight | 165.12 g/mol |
| Synonyms | 5-Fluoro-2,3-indoledione, 5-Fluoroindole-2,3-dione, NSC 39161 |
| Classification / Family | Fluorinated building block, Heterocyclic building block, APIs, Catalysts, OLEDs |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 224 °C – 227 °C |
| Appearance | Deep red powder |
MSDS Documentation
Literature and Reviews
- Cobalt isatin‑Schiff‑base derivative of MOF as a heterogeneous multifunctional bio‑photocatalyst for sunlight‑induced tandem air oxidation condensation process, M. Rouzifar et al., Sci. Rep., 13, 515(2023); DOI: 10.1038/s41598-023-32241-z.
- Computational evaluation and experimental in vitro antibacterial, antifungal and antiviral activity of bis-Schiff bases of isatin and its derivatives, A. Jarrahpour et al., Med. Chem. Res., 22, 1203–1211(2013); DOI: 10.1007/s00044-012-0127-6.
- Recyclable (PhSe)2-Catalyzed selective oxidation of isatin by H2O2: a practical and waste-free access to isatoic anhydride under mile and neutral conditions, L. Yu et al., Catal. Sci. Technol., 5, 4830–4838(2015); DOI: 10.1039/C5CY01030F.
