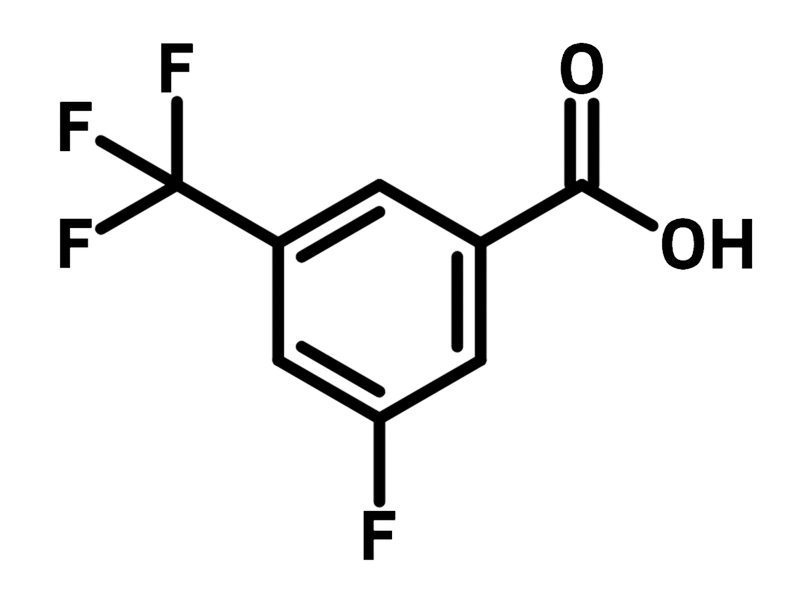
3-Fluoro-5-(trifluoromethyl)benzoic acid
CAS Number 161622-05-5
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers,A fluorinated benzoic acid building block
As a synthesis intermediate for APIs
Specifications | MSDS | Literature and Reviews
3-Fluoro-5-(trifluoromethyl)benzoic acid (CAS number 161622-05-5) is a benzoic acid derivative featuring a fluoride and a trifluoromethyl at the 3- and 5-positions, respectively. 3-Fluoro-5-(trifluoromethyl)benzoic acid is employed in the synthesis of active pharmaceutical ingredient (APIs) due to the excellent lipophilicity and binding affinity provided by the fluorinated substituents. As a benzoic acid building block, 3-fluoro-5-(trifluoromethyl)benzoic acid can be easily attached to molecule scaffolds through amination reactions. The carboxylic acid group can be converted to an acyl chloride for nucleophilic substitution and Friedel-Craft acylation.
A fusion inhibitor of influenza A virus, based on oligothiophene, is capped by 3-fluoro-5-(trifluoromethyl)benzoic acid. The product exhibits an inhibition of 0.22 µM on the membrane fusion between the virus and the endosome of the host cells.
Multiple functional groups
For facile synthesis
Fluorinated benzoic acid building block
For drug discovery, medicinal chemistry, and biochemistry
Low Cost
Competitively priced, high quality product
High purity
>98% High purity
General Information
| CAS Number | 161622-05-5 |
| Chemical Formula | C8H4F4O2 |
| Full Name | 3-Fluoro-5-(trifluoromethyl)benzoic acid |
| Molecular Weight | 208.11 g/mol |
| Synonyms | α,α,α,5-Tetrafluoro-m-toluic acid |
| Classification / Family | Fluorinated building blocks, Benzoic acid building blocks, APIs |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 104 °C – 108 °C |
| Appearance | White Powder |
MSDS Documentation
3-Fluoro-5-(trifluoromethyl)benzoic acid MSDS Sheet
Literature and Reviews
- Antibacterial activity of new dibenzoxepinone oximes with fluorine and trifluoromethyl group substituents, C. Limban et al., Int. J. Mol. Sci., 12, 6432–6444 (2021); DOI: 10.3390/ijms12106432.
- Sterol 14α−Demethylase Structure-Based Design of VNI ((R)-N−(l-(2,4-Dichlorophenyl)-2-(1H−imidazol-1-yl)ethyl)-4-(5-phenyl-1,3,4- oxadiazol-2-yl)benzamide) derivatives to target fungal infections: synthesis, biological evaluation, and crystallographic analysis, L. Friggeri et al., J. Med. Chem., 61 (13), 5679–5691 (2018); DOI: 10.1021/acs.jmedchem.8b00641.
- 4-4-(Anilinomethyl)-3-[4-(trifluoromethyl)phenyl]-1H-pyrazol-1-ylbenzoic acid derivatives as potent anti-gram-positive bacterial agents, R. Hansa et al., Eur. J. Med. Chem., 219, 113402 (2021); DOI: 10.1016/j.ejmech.2021.113402.
