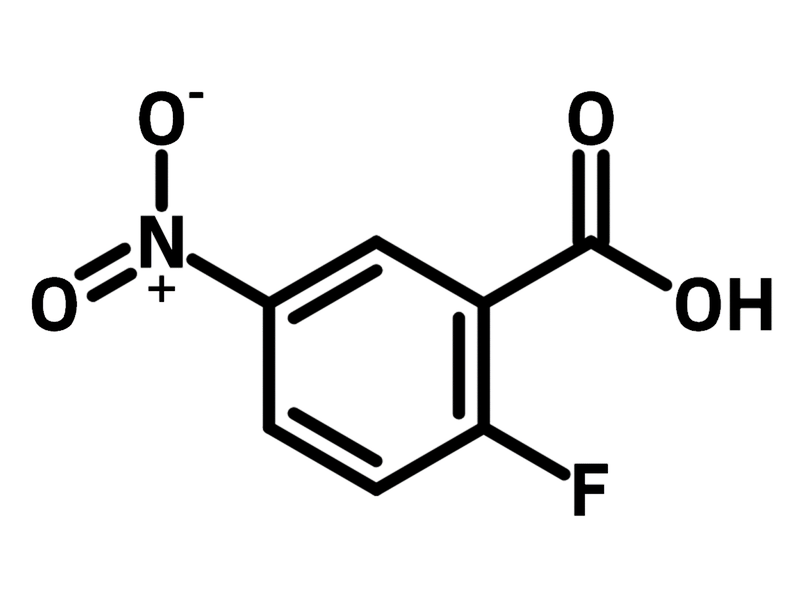
2-Fluoro-5-nitrobenzoic acid
CAS Number 7304-32-7
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials,A fluorinated nitrobenzoic acid building block
Having multiple functional groups for synthesizing fluorescent sensing molecules, heterocyclic APIs and peptides
Specifications | MSDS | Literature and Reviews
2-Fluoro-5-nitrobenzoic acid (CAS number 7304-32-7) is a benzoic acid derivative with substituents of a fluorine and a nitro-group at 2- and 5-positions. Fluorescent probes for detecting nucleophiles such as endogenous hydrogen polysulfide and hydrazine can be synthesized from 2-fluoro-5-nitrobenzoic acid and fluorescent dyes through esterification. The fluorescent probes have intrinsically weak fluorescence, and yet to be turned on by the targeting nucleophiles. The nucleophiles hydrolyse the fluorescent probes (ester hydrolysis), which releases the strong fluorescent dyes observed by fluorescent microscopy.
The fluorine atom substitutes beside the carboxylic group in 2-fluoro-5-nitrobenzoic acid, enabling it to synthesize polycyclic heterocycles and β-turn cyclic peptidomimetics.
Multiple functional groups
For facile synthesis
Fluorinated nitrobenzoic acid building block
For drug discovery, bioimaging and peptide research
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 7304-32-7 |
| Chemical Formula | C7H4FNO4 |
| Full Name | 2-Fluoro-5-nitrobenzoic acid |
| Molecular Weight | 185.11 g/mol |
| Synonyms | 2-Fluoro-5-nitrobenzenecarboxylate |
| Classification / Family | Fluorinated building block, fluorescent probes, Heterocyclics, APIs, Peptides |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 142 °C – 144 °C |
| Appearance | White powder |
MSDS Documentation
2-Fluoro-5-nitrobenzoic acid MSDS Sheet
Literature and Reviews
- Efficient solid-phase synthesis of 2,1,3-benzothiadiazin-4-one 2-oxides with synphaseTM lanterns, S. Makino et al., Bull. Korean Chem. Soc., 24(3), 389–392(2003); DOI: 10.5012/bkcs.2003.24.3.389.
- One-pot high-throughput synthesis of β-turn cyclic peptidomimetics via "volatilizable" supports, Y. Li et al., J. Org. Chem., 74(5), 2183–2185(2009); DOI:10.1021/jo802583t.
- Synthesis and the crystal structure of (E)-2-(7-(3-(thiophen-2-yl)acrylamido)-2,3-dihydro-5-oxobenzo[e][1,4]oxazepin-1(5H)-yl) ethyl acetate, Y Lee et al., J. Chem. Crystallogr., 39, 902–907(2009); DOI: 10.1007/s10870-009-9588-y.
