6-Fluoropyridine-2-carbonitrile
CAS Number 3939-15-9
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials,A fluorinated pyridine building block
For the synthesis of metal-organic complexes and APIs
Specifications | MSDS | Literature and Reviews
6-Fluoropyridine-2-carbonitrile (CAS number 3939-15-9) is a pyridine derivative substituted with a nitrile and a fluorine at the two ortho-positions. 6-Fluoropyridine-2-carbonitrile can form a centrosymmetric dinuclear complex with silver (AgI). The complex exhibits a molecular ladder-like structure linked by the BF4- counteranion via Ag-F interactions. 6-Fluoropyridine-2-carbonitrile can be attached to molecular scaffolds effectively through nucleophilic aromatic substitution.
The nitrile group in 6-fluoropyridine-2-carbonitrile reacts with thiols or amines to produce thiazoles and imidazoles. These heterocyclic compounds are widely used as active pharmaceutical ingredients (APIs).
Multiple functional groups
For facile synthesis
Fluorinated pyridine building block
for drug discovery, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
General Information
| CAS Number | 3939-15-9 |
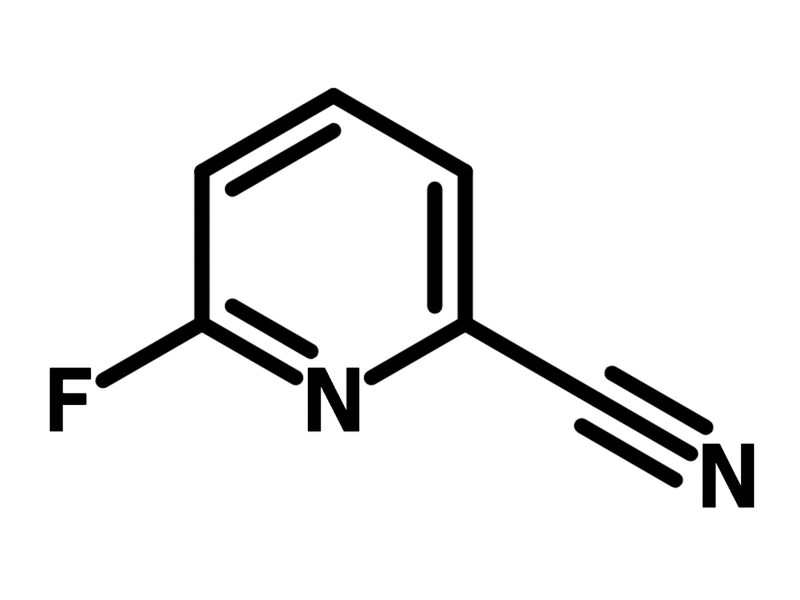
| Chemical Formula | C6H3FN2 |
| Full Name | 6-Fluoropyridine-2-carbonitrile |
| Molecular Weight | 122.10 g/mol |
| Synonyms | 2-Cyano-6-fluoropyridine |
| Classification / Family | Fluorinated building blocks, Heterocyclic building blocks, Ligands, APIs |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | Tm = 31 °C – 35 °C |
| Appearance | Pale blue crystals |
MSDS Documentation
6-Fluoropyridine-2-carbonitrile MSDS Sheet
Literature and Reviews
- Bis(l-2-cyanopyridine-N:N')bis-[(2-cyanopyridine-N)silver(I)]bis(tetrafluoroborate): an anion-linked molecular ladder, A. Blake et al., Acta Cryst., C57, 1290–1291(2001); ISSN 0108-2701.
- Characterization of N-glucuronidation of 4-(5-pyridin-4-yl-1H-[1,2,4]triazol-3-yl)pyridine-2-carbonitrile (FYX-051): a new xanthine oxidoreductase inhibitor, K. Omura et al., Drug Metab. Dispos., 35(12), 2143–2148(2007); DOI: 10.1124/dmd.107.017251.
- Biocompatible macrocyclization between cysteine and 2-cyanopyridine generates stable peptide inhibitors, C. Nitsche et al., Org. Lett., 21(12), 4709–4712(2019); DOI: 10.1021/acs.orglett.9b01545.
