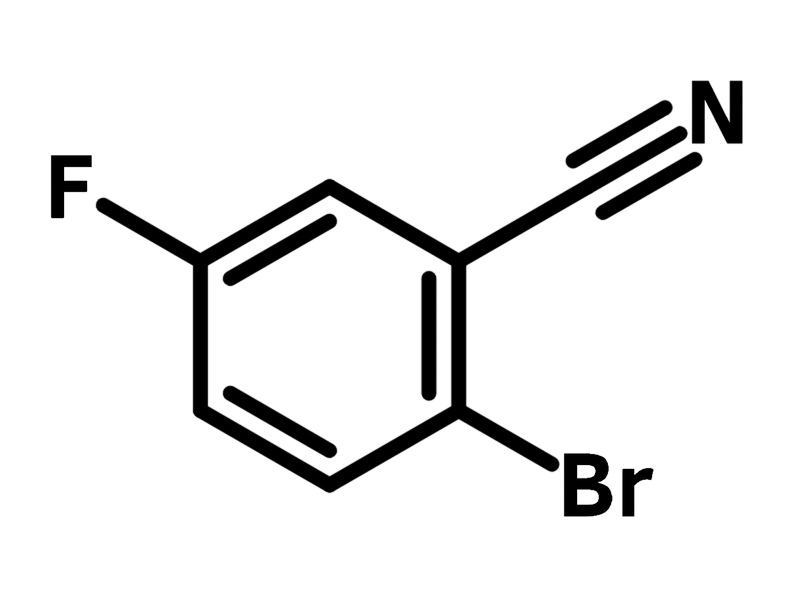
2-Bromo-5-fluorobenzonitrile
CAS Number 57381-39-2
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers,A dihalogenated benzonitrile building block
Used as a precursor for the synthesis of TADF dyes in OLED applications and APIs in antitumour and anti-inflammatory applications.
Specifications | MSDS | Literature and Reviews
2-Bromo-5-fluorobenzonitrile (CAS number 57381-39-2) is a benzonitrile derivative, bearing a bromide and a fluoride at the 2- and 5-positions. The bromide and fluoride substituents display different reactivities, thus enabling selective substitution reactions. Fluoride favors nucleophilic aromatic substitution, while bromide is amenable to Pd-catalyzed coupling reactions. A thermally activated delayed fluorescence (TADF) dye, named as 2-phenoxazine-5-acridine-benzonitrile, is synthesized from 2-bromo-5-fluorobenzonitrile with phenoxazines, carbazoles or acridan in a two-step reaction that includes nucleophilic aromatic substitution and Buchwald-Hartwig amination. The resulting OLED device shows a maximum current efficiency of 16.3 cdA-1, a maximum powder efficiency of 12.2 lmW-1 and an external quantum efficiency of 5%.
The ortho positioning of bromide and nitrile groups of 2-bromo-5-fluorobenzonitrile facilitates its synthesis into quinazolines for the use in antitumour and anti-inflammatory applications.
Multiple functional groups
For facile synthesis
Fluorinated benzonitrile building block
For drug discovery, TADF dyes, OLEDs research
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 57381-39-2 |
| Chemical Formula | C7H3BrFN |
| Full Name | 2-Bromo-5-fluorobenzonitrile |
| Molecular Weight | 200.01 g/mol |
| Synonyms | N/A |
| Classification / Family | Fluorinated building blocks, Benzonitrile building blocks, TADF, OLEDs, APIs |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 92 °C – 95 °C |
| Appearance | White powder/crystals |
MSDS Documentation
2-Bromo-5-fluorobenzonitrile MSDS Sheet
Literature and Reviews
- Differently substituted benzonitriles for non-doped OLEDs, D. Gudeika et al., Dyes Pigm., 172, 107789(2020); DOI: 10.1016/j.dyepig.2019.107789.
- "On-water" synthesis of quinazolinones and dyhydroquinazolinones starting from o-bromobenzonitrile, Z. Liu et al., Molecules, 23, 2325(2018); DOI: 10.3390/molecules23092325.
- Substrate profiling of the cobalt nitrile hydratase from Rhodococcus rhodochrous ATCC bAA 870, A. Mashweu, Molecules, 25, 238(2020); DOI: 10.3390/molecules25010238.
