4′-Fluoro-3′-nitroacetophenone
CAS Number 400-93-1
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers,A fluorinated acetophenone building block
As a synthesis intermediate for APIs
Specifications | MSDS | Literature and Reviews
4′-Fluoro-3′-nitroacetophenone (CAS number 400-93-1) is a derivative of acetophenone with fluoride and nitro substituents. With its three functional groups, 4′-fluoro-3′-nitroacetophenone commonly serves as a molecular scaffold for active pharmaceutical ingredients. An inhibitor of Trypanosoma cruzi (Tc, causing Chagas disease) is synthesized from 4′-fluoro-3′-nitroacetophenone through a series of reactions, including nucleophilic aromatic substitution on the fluoride group, amination on the ketone and reduction on the nitro group. The resulting product shows promising efficacy in Tc infection with a potency of 8 nM.
4′-Fluoro-3′-nitroacetophenone is also utilized for the synthesis of bicyclic heterocycles, such as chromen derivatives.
Multiple functional groups
For facile synthesis
Fluorinated nitroacetophenone building block
For drug discovery, medicinal chemistry, and biochemistry
Low Cost
Competitively priced, high quality product
High purity
>98% High purity
General Information
| CAS Number | 400-93-1 |
| Chemical Formula | C8H6FNO3 |
| Full Name | 1-(4-Fluoro-3-nitrophenyl)ethanone |
| Molecular Weight | 183.14 g/mol |
| Synonyms | 3-Acetyl-6-fluoronitrobenzene, 4-Acetyl-1-fluoro-2-nitrobenzene |
| Classification / Family | Fluorinated building blocks, Nitro building blocks, Acetophenone building blocks, APIs |
Chemical Structure
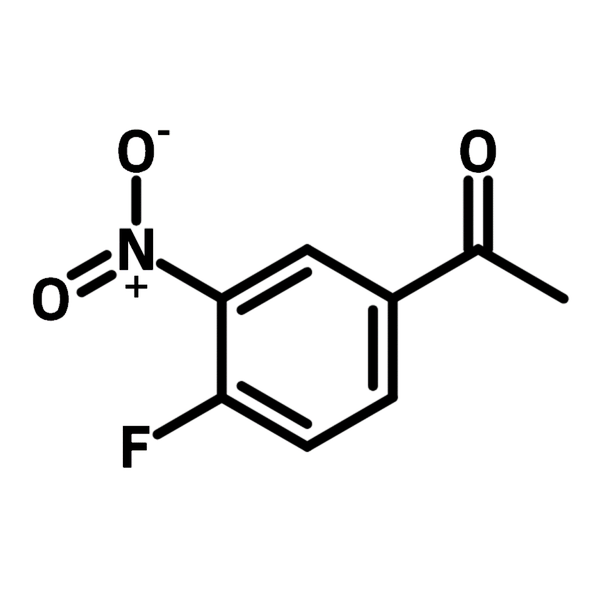
Product Details
| Purity | 98% |
| Melting Point | Tm = 47 °C – 51 °C |
| Appearance | Light brown crystals |
MSDS Documentation
4′-Fluoro-3′-nitroacetophenone MSDS Sheet
Literature and Reviews
- Synthesis, biological evaluation and structure-activity relationship of novel dichloroacetophenones targeting pyruvate dehydrogenase kinases with potent anticancer activity, B. Xu et al., Eur. J. Med. Chem., 214, 113225 (2021); DOI: 10.1016/j.ejmech.2021.113225.
- Synthesis and anticancer study of novel 4H-chromen derivatives, X. Lu et al., Anticancer Agents Med. Chem., 17 (8), 1070–1083 (2023); DOI: 10.2174/1871520615666160504094945.
- Defect-mediated selective hydrogenation of nitroarenes on nanostructured WS2, Y. Sun et al., Chem. Sci., 10, 10310 (2019); DOI: 10.1039/c9sc03337h.
