Pentafluorobenzoic acid
CAS Number 602-94-8
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers,A perfluorinated benzoic acid building block
Forms organometallic complexes for catalysts and APIs
Specifications | MSDS | Literature and Reviews
Pentafluorobenzoic acid (CAS number 602-94-8), also known as perfluorobenzoic acid, is a benzoic acid compound that is fully substituted with five fluorine groups on the benzene ring. Being a perfluorinated compound, pentafluorobenzoic acid is considered to be electron deficient. It finds application in the exploration of the reaction mechanism involved in indolyl 1,3-heteroatom transposition reactions.
Pentafluorobenzoic acid can coordinate to metal centers, forming organometallic complexes. It acts as a bidentate ligand for the preparation of 2D coordination polymers with copper and fullerene. Density functional theory (DFT) calculation reveals that a pentafluorobenzoic acid-copper(I)-fullerene complex possesses a narrow bandgap of 0.95 eV. Additionally, by taking the advantage of the electron deficiency, pentafluorobenzoic acid is utilized for the study of the π-stacking interaction in titanium salalen catalysts.
Multiple functional groups
For facile synthesis
Fluorinated benzoic acid building block
for drug discovery, organometallic complexes, and organic synthesis
Low Cost
Competitively priced, high quality product
High purity
>98% High purity
General Information
| CAS Number | 602-94-8 |
| Chemical Formula | C7HF5O2 |
| Full Name | Pentafluorobenzoic acid |
| Molecular Weight | 212.08 g/mol |
| Synonyms | Perfluorobenzoic acid |
| Classification / Family | Fluorinated building blocks, Benzoic acid building blocks, Catalysts, Organometallic complexes, Organic synthesis, APIs |
Chemical Structure
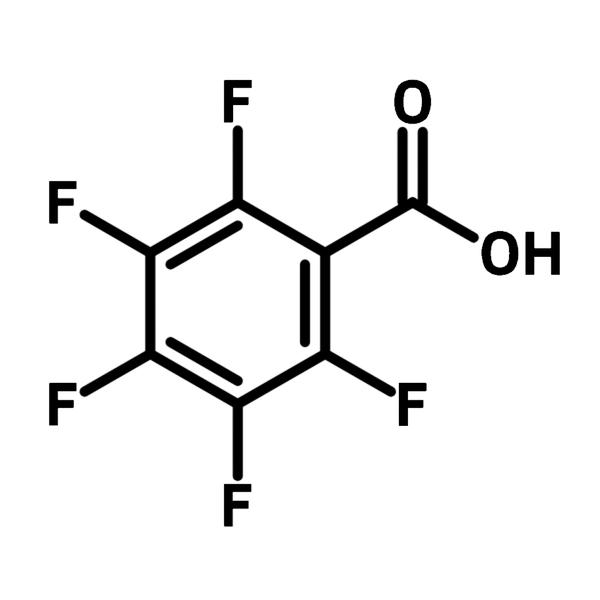
Product Details
| Purity | 98% |
| Melting Point | Tm = 100 °C – 102 °C |
| Appearance | White powder |
MSDS Documentation
Pentafluorobenzoic acid MSDS Sheet
Literature and Reviews
- Titanium salalen catalyzed enantioselective benzylic hydroxylation, C. Wartmann et al., Angew. Chem. Int. Ed., 62, e202306584 (2023); DOI: 10.1002/anie.202306584.
- Mechanistic duality of indolyl 1,3-heteroatom transposition, Y. Lee et al., Chem. Sci., 14, 7688 (2023); DOI: 10.1039/d3sc00716b.
- Protonation behavior of a tetrahydrido molybdenum(IV) complex with organic and inorganic acids, N. Queyriaux et al., Eur. J. Inorg. Chem., 26, e202300426 (2023); DOI: 10.1002/ejic.20230042.
