BDTT-F-Sn
CAS Number 1514905-25-9
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers,High purity BDTT-F-Sn, for applications in highly efficient OSCs and OPVs
For the synthesis of small molecular donors and low band gap semiconducting polymers
Specifications | MSDS | Literature and Reviews | Related Products
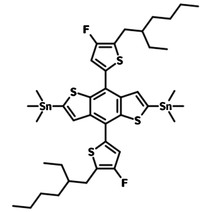
2,6-bis(trimethytin)-4,8-bis(5-(2-ethylhexyl)-4-fluorothiophen-2-yl)benzo[1,2-b:4,5-b’]dithiophene (BDTT-FS-n), CAS number 1514905-25-9, is an electron-rich building block used for the synthesis of either small molecular donors or low band-gap polymer semiconductors (like PBDB-T-F) for highly-efficient OSCs and OPV applications.
Bearing two ethylhexyl side chains, with fused benzodithiophenes and thiophenes as electron-rich pendants, BDTT-F-Sn offers good solubility for the targeted materials. The introduction of the fluoro atoms on the side thiophene rings can also lower the HOMO/LUMO energy levels of the desired polymers.
Available to purchase up to 25 g. If you are ordering the item with quantity over 5 g please contact our customer care team for a lead time.
Capped with trimethyltin
for facile coupling reactions
Worldwide shipping
Quick and reliable shipping
Benzodithiophene building block
For semiconductors, OFETs, and solar cells
High purity
>98% High purity
General Information
| CAS Number | 1514905-25-9 |
| Chemical Formula | C40H56F2S4Sn2 |
| Molecular Weight | 940.55 g/mol |
| Full Name | 2,6-bis(trimethytin)-4,8-bis(5-(2-ethylhexyl)-4-fluorothiophen-2-yl)benzo[1,2-b:4,5-b’]dithiophene |
| Synonyms |
|
| Classification / Family | Thiophene, Fused thiophene, Benzo-dithiophene heterocylic aromatics, Five-membered ring, Semiconductor synthesis intermediates, Low band-gap polymers, OFETs, Organic photovoltaics, Polymer solar cells |
Chemical Structure
Product Details
| Purity | >98% (by 1H-NMR) |
| Melting Point | N/A |
| Appearance | Light yellow crystals |
MSDS Documentation
Literature and Reviews
- Over 14% Efficiency in Polymer Solar Cells Enabled by a Chlorinated Polymer Donor, S. Zhang et al., Adv.Mater., 30, 1800868 (2018); DOI: 10.1002/adma.201800868.
- Design of a New Small‐Molecule Electron Acceptor Enables Efficient Polymer Solar Cells with High Fill Factor, S. Li et al., Adv.Mater., 29, 1704051 (2017); DOI: 10.1002/adma.201704051.
- A High-Efficiency Organic Solar Cell Enabled by the Strong Intramolecular Electron Push–Pull Effect of the Nonfullerene Acceptor, W. Li et al., Adv.Mater., 30, 1707170 (2017); DOI: 10.1002/adma.201707170.


