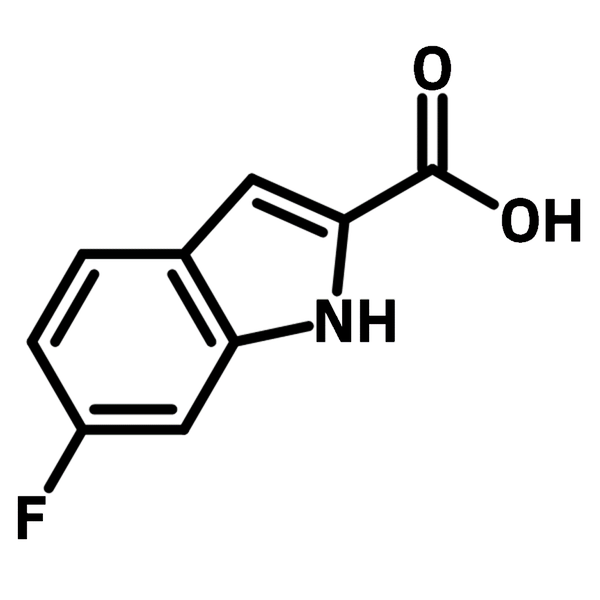
6-Fluoroindole-2-carboxylic acid
CAS Number 3093-97-8
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials,A fluorinated indole with a carboxylic acid anchoring group
Used as a synthesis intermediate for metal complexes, macromolecules, and bioactive compounds in application of APIs, solar cells and OFETs
Specifications | MSDS | Literature and Reviews
6-Fluoroindole-2-carboxylic acid (CAS number 3093-97-8) is a fluorinated indole derivative, having a fluorine at 6-position and a carboxylic acid at 2-position. The secondary amine and carboxylic acid in 6-Fluoroindole-2-carboxylic acid form an α-amino acid moiety. This allows 6-fluoroindole-2-carboxylic acid to have a zwitterionic form where the proton of the carboxylic acid is transferred to the amine. In fluorescence spectra, the neutral form and zwitterionic form of 6-fluoroindole-2-carboxylic acid have different emission band at 332 and 370 nm respectively, forming a broad fluorescence emission peak.
The carboxylic acid group in 6-fluoroindole-2-carboxylic acid can bind to copper(II), yielding a binuclear complex. The complex shows anti-cancer activity towards breast cancer cell lines (MDA-MB-231 and MCF-7) with percentage of inhibition of 90% at 20 µM concentration.
6-Fluoroindole-2-carboxylic acid can be used to synthesize triindoles, via a palladium catalyzed decarboxylative reaction. Triindoles are used as semiconductors in OFETs with field effect mobility of 0.03 cm2V-1S-1, as well as hole transport layers in solar cells.
Multiple functional groups
For facile synthesis
Fluorinated indole building block
For drug discovery, solar cells, and OLEDs
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 3093-97-8 |
| Chemical Formula | C9H6FNO2 |
| Full Name | 6-Fluoro-1H-indole-2-carboxylic acid |
| Molecular Weight | 179.15 g/mol |
| Synonyms | N/A |
| Classification / Family | Fluorinated building block, Heterocyclic building block, APIs, OFETs, Solar cells, Semiconductors |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | Tm = 244 °C – 250 °C |
| Appearance | White to off-white powder |
MSDS Documentation
6-Fluoroindole-2-carboxylic acid MSDS Sheet
Literature and Reviews
- Excited state proton transfer in indole-2-carboxylic acid and indole-5-carboxylic acid, P. Bangal et al., J. Phys. Chem. A, 103, 8585-8594(1999); DOI: 10.1021/jp991884f.
- Halogenated indoles decease the virulence of Vibrio campbellii in a gnotobiotic brine shrimp model, S. Zhang et al., Microbiol. Spectr., 10(5), e02689-22(2022); DOI: 10.1128/spectrum.02689-22.
- In vitro DNA-binding, antioxidant and anticancer anctivity of indole-2-carboxylic acid dinuclear copper(II) complex, X. Wang et al., Molecules, 22, 171(2017); DOI: 10.3390/molecules22010171.
