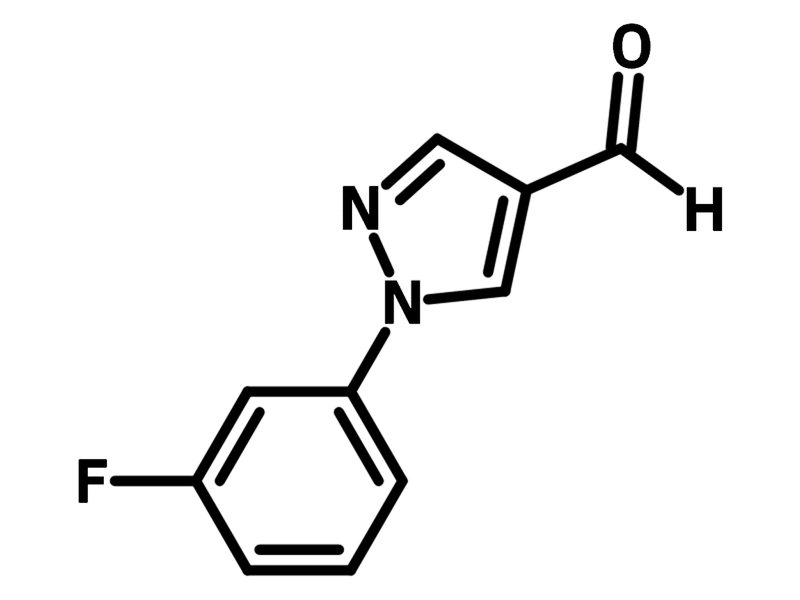
1-(3-Fluorophenyl)-1H-pyrazole-4-carbaldehyde
CAS Number 936940-82-8
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials,A fluorinated phenylpyrazole building block
Used as a reaction intermediate for synthesizing APIs
Specifications | MSDS | Literature and Reviews
1-(3-Fluorophenyl)-1H-pyrazole-4-carbaldehyde (CAS number 936940-82-8) is a pyrazole derivative substituted with a 3-fluorophenyl and an aldehyde group. Pyrazole derivatives are widely utilized as active pharmaceutical ingredients (APIs) including celecoxib. A novel anti-inflammatory pyrazole derivative, LQFM021, incorporates 1-(3-fluorophenyl)-1H-pyrazole-4-carbaldehyde in its synthesis. The entire synthesis process takes two steps: the functional group conversion of the aldehyde group to a nitrile and the formation of a tetrazole using sodium azide.
1-(3-Fluorophenyl)-1H-pyrazole-4-carbaldehyde is also employed in the synthesis of a pyrazole-based disubstituted urea, serving as an anti-melanoma agent.
Multiple functional groups
For facile synthesis
Fluorinated phenylpyrazole building block
For drug discovery, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 936940-82-8 |
| Chemical Formula | C10H7FN2O |
| Full Name | 1-(3-Fluorophenyl)-1H-pyrazole-4-carbaldehyde |
| Molecular Weight | 190.17 g/mol |
| Synonyms | N/A |
| Classification / Family | Fluorinated building blocks, Heterocyclic building blocks, Aldehyde building blocks, APIs |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | N/A |
| Appearance | Brown powder/crystals |
MSDS Documentation
1-(3-Fluorophenyl)-1H-pyrazole-4-carbaldehyde MSDS Sheet
Literature and Reviews
- Modification, biological evaluation and SAR studies of novel 1H-pyrazole derivatives containing N,N'-disubstituted urea moiety as potential anti-melanoma agents, B.-F. Ruan et al., Chem. Biodiversity, 15, e1700504(2018); DOI: 10.1002/cbdv.201700504.
- In vitro genotoxicity and in vivo subchronic evaluation of the anti inflammatory pyrazole compound LQFM021, S. de Moura et al., Chem. Biol. Interact., 277, 185–194(2017); DOI: 10.1016/j.cbi.2017.09.004.
- Synthesis, characterization, and antimicrobial evaluation of carbostyril derivatives of 1H-pyrazole, SPJ, 19, 75–83(2011); DOI: 10.1016/j.jsps.2011.01.005.
