trans-3,4-Difluorocinnamic acid
CAS Number 112897-97-9
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Non-Heterocyclic Building BlocksA fluorinated cinnamic acid building block
Used as a synthesis precursor for APIs
Specifications | MSDS | Literature and Reviews
trans-3,4-Difluorocinnamic acid (CAS number 112897-97-9) is a difluoro-substituted benzene featuring a trans-propenoic acid. trans-3,4-Difluorocinnamic acid is readily used for the synthesis of substituted isoquinolones through Curtius rearrangement. Substituted isoquinolones are found to be effective 5-HT3 antagonists (infective dose ID50 of 0.35 μg/kg) in anticancer treatments.
trans-3,4-Difluorocinnamic acid is also utilized in the synthesis of psammaplin A derivatives as radiosensitizers for human lung cancer. The product derived from trans-3,4-difluorocinnamic acid demonstrates a potency of 16.14 μM.
Multiple functional groups
For facile synthesis
Fluorinated cinnamic acid building block
For drug discovery, medicinal chemistry, and biochemistry
Low Cost
Competitively priced, high quality product
High purity
>98% High purity
General Information
| CAS Number | 112897-97-9 |
| Chemical Formula | C9H6F2O2 |
| Full Name | (2E)-3-(3,4-Difluorophenyl)-prop-2-enoic acid |
| Molecular Weight | 184.14 g/mol |
| Synonyms | (2E)-3-(3,4-Difluorophenyl)acrylic acid |
| Classification / Family | Fluorinated building blocks, Cinnamic acid building blocks, APIs |
Chemical Structure
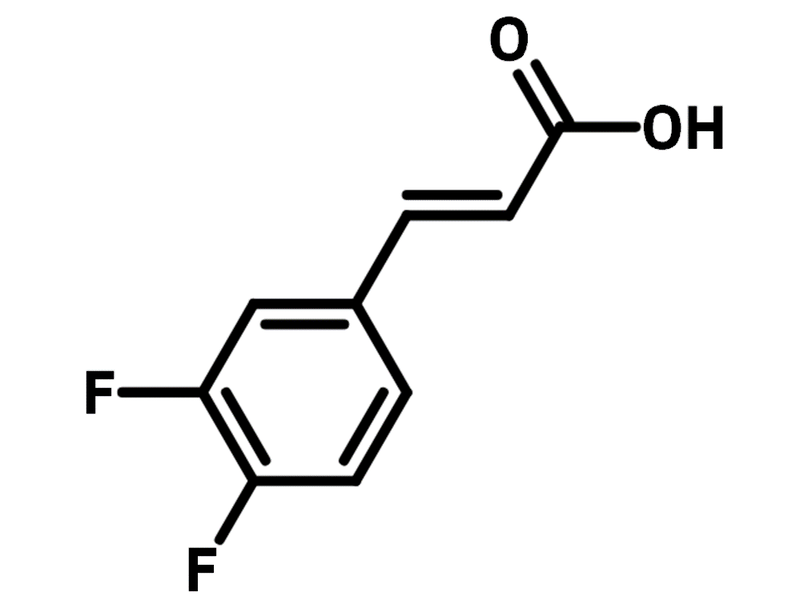
Product Details
| Purity | 98% |
| Melting Point | Tm = 194 °C – 196 °C |
| Appearance | White powder |
MSDS Documentation
trans-3,4-Difluorocinnamic acid MSDS Sheet
Literature and Reviews
- Synthesis of novel substituted isoquinolones, N. Briet et al., Tetrahedron, 58, 5761–2766 (2002); DOI: 10.1016/S0040-4020(02)00573-2.
- Harmicines — harmine and cinnamic acid hybrids as novel antiplasmodial hits, I. Perković et al., Eur. J. Med. Chem., 187, 111927 (2020); DOI: 10.1016/j.ejmech.2019.111927.
- Synthesis of unnatural flavonoids and stilbenes by exploiting the plant biosynthetic pathway in Escherichia coli, Y. Katsuyama et al., Chem. Biol., 14, 613–621 (2007); DOI: 10.1016/j.chembiol.2007.05.004.
