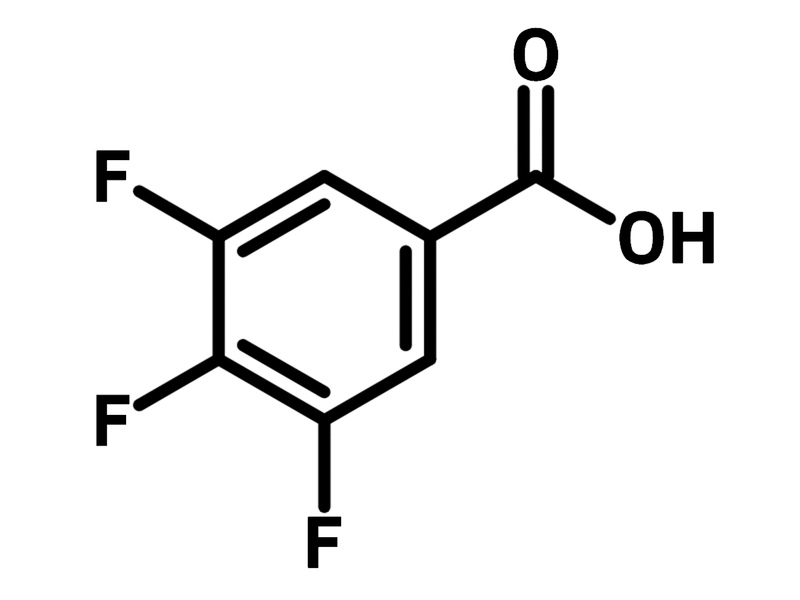
3,4,5-Trifluorobenzoic acid
CAS Number 121602-93-5
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers,A polyfluorinated benzoic acid building block
Applied as a transient directing group in catalytic C-H activation and a building block for APIs
Specifications | MSDS | Literature and Reviews
3,4,5-Trifluorobenzoic acid (CAS number 121602-93-5) is a benzoic acid derivative with three fluorine substituents at para- and meta-positions. 3,4,5-Trifluorobenzoic acid finds its application as a transient directing group in transition metal catalyzed C-H activation reactions. The fluorine electron withdrawing groups contribute the ideal acidity to the carboxylic acid while no steric hindrance group is present at the ortho-positions. 3,4,5-Trifluorobenzoic acid assists the concerted metalation deprotonation as the C-H activation step, thus facilitating the catalytic cycle.
A salt of 3,4,5-trifluorobenzoic acid improves the solubility and permeability of naftopidil in benign prostatic hyperplasia treatment. 3,4,5-Trifluorobenzoic acid is also used as a synthetic building block for dibenzoate esters type anticancer drugs.
Multiple functional groups
For facile synthesis
Fluorinated benzoic acid building block
For organic synthesis, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 121602-93-5 |
| Chemical Formula | C7H3F3O2 |
| Full Name | 3,4,5-Trifluorobenzoic acid |
| Molecular Weight | 176.09 g/mol |
| Synonyms | N/A |
| Classification / Family | Fluorinated building block, APIs, Transiet directing groups, Reaction additives, C-H activation |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 97 °C – 99 °C |
| Appearance | White crystals |
MSDS Documentation
3,4,5-Trifluorobenzoic acid MSDS Sheet
Literature and Reviews
- PdII-catalyzed C(alkenyl)-H activation facilitated by a transient directing group, M. Liu et al., Angew. Chem. Int. Ed., 61, e202203624(2022); DOI: 10.1002/anie.202203624.
- Dibenzoate esters of cis-tetralin-2,3-diol as analogs of (−)-epigallocatechin gallate: synthesis and crystal structure of anticancer drug candidates, R. Rutherford et al., Acta Cryst., C76, 1085–1095(2020); DOI: 10.1107/S2053229620014916.
- Fluorobenzoic acid coformers to improve the solubility and permeability of the BCS class IV drug naftopidil, M. Mannava et al., Chem. Commun., 58, 5582–5585(2022); DOI: 10.1039/D1CC07187D.
