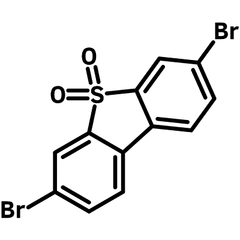
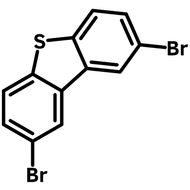
3,7-Dibromodibenzothiophene 5,5-dioxide
CAS Number 83834-12-2
Chemistry Building Blocks, Dibromo Monomers, Heterocyclic Building Blocks, Monomers
High-Purity 3,7-Dibromodibenzothiophene 5,5-dioxide
A popular intermediate for the further synthesis of oligomers and polymers in the application organic electronics and hydrogen evolution reaction (HER).
3,7-Dibromodibenzothiophene 5,5-dioxide (DBTDO), CAS number 83834-12-2, has a chemical structure of 4,4'-dibromo-1,1'-biphenyl with a Sulfonyl (SO2) bridge. With a structure that is topologically similar to that of fluorene, dibenzothiophene-S,S-dioxide is an electron-deficient moiety due to the electron-withdrawing character of the SO2 group.
With a rigid structure and excellent electron deficiency, 3,7-dibromodibenzothiophene 5,5-dioxide has been widely used as the building block for the electron acceptor moiety to form D–A type conjugated oligomers or polymers with high photocatalytic activity. In addition, due to the good hydrophilic nature of the dibenzothiophene dioxide group, the introduction can improve the dispersion of the targeting function materials in the aqueous solution, thereby improving the photocatalytic hydrogen evolution activity.
3,7-dibromodibenzothiophene 5,5-dioxide is prepared by brominating dibenzothiophene 5,5-dioxide with N-Bromosuccinimide (NBS) in concentrated sulfuric acid.
General Information
| CAS Number | 83834-12-2 |
| Chemical Formula | C12H6Br2O2S |
| Full Name | 3,7-Dibromodibenzothiophene 5,5-dioxide |
| Molecular Weight | 374.05 g/mol |
| Synonyms |
3,7-Dibromodibenzothiophene Sulfone 3,7-Dibromodibenzothiophene S,S-dioxide DBTDO |
| Classification / Family | Dibromodibenzothiophene, semiconductor synthesis intermediates, low band gap polymers, OLED, OFETs, organic photovoltaics |
Chemical Structure

Product Details
| Purity | >99% (HPLC) |
| Melting Point | Tm = 312 °C |
| Appearance | White to off-white powder/crystals |
MSDS Documentation
 3,7-Dibromodibenzothiophene 5,5-dioxide MSDS sheet
3,7-Dibromodibenzothiophene 5,5-dioxide MSDS sheet
Literature and Reviews
-
Synthesis of novel thiophene-phenylene oligomer derivatives with a dibenzothiophene-5,5-dioxide core for use in organic solar cells, S. Fujii et al., Phys. Status Solidi B, 249 (12), 2648-2651 (2012); DOI: 10.1002/pssb.201200439.
-
Conjugated donor-acceptor polymer photocatalysts with electron-output “tentacles” for efficient hydrogen evolution, Z. Lan et al., Appl. Catal. B: Environ., 245 (15), 596-603 (2019); DOI: 10.1016/j.apcatb.2019.01.010.
-
Dibenzothiophene Dioxide Based Conjugated Microporous Polymers for Visible-Light-Driven Hydrogen Production, Z. Wang et al., ACS Catal., 8 (9), 8590–8596 (2018); 10.1021/acscatal.8b02607.
-
Dibenzothienobisbenzothiophene-a novel fused-ring oligomer with high field-effect mobility, H. Sirringhaus et al., J. Mater. Chem., 9, 2095-2101 (1999); DOI: 10.1039/A902679G.
-
Effect of D/A Ratio on Photocatalytic Hydrogen Evolution Performance of Conjugated Polymer Photocatalysts, H. Zhao et al., ACS Appl. Energy Mater., 5, 4, 4631–4640 (2022); DOI: 10.1021/acsaem.2c00017.
-
Engineering Nanoparticulate Organic Photocatalysts via a Scalable Flash Nanoprecipitation Process for Efficient Hydrogen Production, M. Yu et al., Angew. Chem. Int. Ed., 60 (28), 15590-15597 (2021); DOI: 10.1002/anie.202104233.
-
Construction of polymeric carbon nitride and dibenzothiophene dioxide-based intramolecular donor–acceptor conjugated copolymers for photocatalytic H2 evolution, F. Yu et al., Nanoscale Adv., 3, 1699-1707 (2021); DOI: 10.1039/D0NA01011A.
- Dibenzothiophene-S,S-Dioxide-Based Conjugated Polymers: Highly Efficient Photocatalyts for Hydrogen Production from Water under Visible Light, C. Dai et al., Small, 14 (34), 1801839 (2018); DOI: 10.1002/smll.201801839.
Related Products
We stock a wide range of monomers available to purchase online. Please contact us if you cannot find what you are looking for.
![2-Ethylhexyl 4,6-dibromo-3-fluorothieno[3,4-b]thiophene-2-carboxylate](http://www.ossila.com/cdn/shop/products/ptb7-monomer-b361-ossila-chemical-structure.png?v=1648818400&width=190)
![2,5-bis(trimethylstannyl)-thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/2_5-bis-trimethylstannyl-thieno-3_2-b-thiophene_structure.jpg?v=1504193831&width=190)
![2,6-dibromo-4,4-bis(2-ethylhexyl)-4H-cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/4Hcyclopentadithiophene.jpg?v=1431610575&width=190)
![2,7-Dibromo-9,9-bis[3,3'-(N,N-dimethylamino)-propyl]fluorene](http://www.ossila.com/cdn/shop/products/dibromo-fluorene-diyl-bisdimethylpropan-amine.jpg?v=1431610994&width=190)
![3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione](http://www.ossila.com/cdn/shop/products/bisbromothiophenyl-bisoctyldodecylpyrrolo-dione.jpg?v=1431611190&width=190)
![4H-Cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/cyclopentadithiophene_12a14774-f96a-4ebc-a0d0-2c5483da9180.jpg?v=1445441165&width=190)
![Benzo[1,2-b:4,5-b']dithiophene-4,8-dione](http://www.ossila.com/cdn/shop/products/benzo-dithiophene-dione.jpg?v=1437904702&width=190)
![Thienothiophene, Thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/thienothiophene.jpg?v=1431611114&width=190)
![Thieno[3,2-b]thiophene-2-carbonitrile](http://www.ossila.com/cdn/shop/products/thienothiophene-2-carbonitrile.jpg?v=1439548051&width=190)
![DTT, Dithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/dtt-chemical-structure.png?v=1653477307&width=190)
![3,6-Dibromothieno[3,2-b]thiophene (TT36)](http://www.ossila.com/cdn/shop/products/3-6-dibromothienothiophene-chemical-structure.png?v=1653663075&width=190)
![2,3,5,6-Tetrabromothieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/Tetrabromo-thienothiophene-chemical-structure.png?v=1665673773&width=190)
![2,6-Dibromodithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/Dibromodithienothiophene-chemical-structure.png?v=1666702461&width=190)
![2,5-Dihydro-3,6-di-2-thienyl-pyrrolo[3,4-c]pyrrole-1,4-dione](http://www.ossila.com/cdn/shop/products/2_5-Dihydro-3_6-di-2-thienyl-pyrrolo_3_4-c_pyrrole-1_4-dione-chemical-structure-dpp.png?v=1667321819&width=190)
![6,9-bis(5-bromo-4-(2-butyloctyl)thiophen-2-yl)dithieno[3,2-f:2',3'-h]quinoxaline](http://www.ossila.com/cdn/shop/products/bisbromo-butyloctylthiophenyl-dithienoquinoxaline-chemical-structure.png?v=1669202898&width=190)
![Indolo[3,2-b]carbazole](http://www.ossila.com/cdn/shop/products/Indolocarbazole-chemical-structure.png?v=1670495077&width=190)
![10,15-Dihydro-5H-diindolo[3,2-a:3',2'-c]carbazole](http://www.ossila.com/cdn/shop/products/Dihydro-diindolocarbazole-chemical-structure.png?v=1670502109&width=190)
![Indolo[2,3-a]carbazole](http://www.ossila.com/cdn/shop/products/indolo-2-3-a-carbazole-chemical-structure-title.png?v=1678288567&width=190)
![2,2-Bis[4-(4-aminophenoxy)phenyl]propane (BAPP)](http://www.ossila.com/cdn/shop/products/bapp-chemical-structure-title.png?v=1679403349&width=190)
![2,2'-Dimethyl[1,1'-biphenyl]-4,4'-diamine](http://www.ossila.com/cdn/shop/products/2-2-dimethyl1-1-biphenyl-4-4-diamine-chemical-structure-title.png?v=1680597662&width=190)
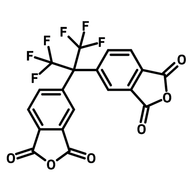
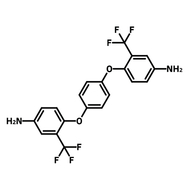
![2,2-Bis[4-(4-aminophenoxy)phenyl]hexafluoropropane (4-BDAF)](http://www.ossila.com/cdn/shop/products/4-bdaf-chemical-structure-title.png?v=1681225583&width=190)
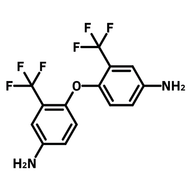
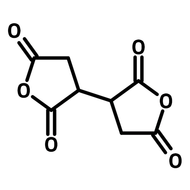
![1-[2-(Trifluoromethyl)phenyl]imidazole](http://www.ossila.com/cdn/shop/files/1-2-trifluoromethylphenylimidazole-chemical-structure-title.png?v=1682593257&width=190)