Thieno[3,2-b]thiophene-2-carbonitrile
CAS Number 40985-58-8
Chemistry Building Blocks, Heterocyclic Building Blocks, Materials, MonomersThieno[3,2-b]thiophene-2-carbonitrile, high purity monomer
Used to adjust the bandgap of organic polymer semiconducting materials
Specifications | Literature and Reviews
Thieno[3,2-b]thiophene-2-carbonitrile (CAS number 40985-58-8), also known as 2-Cyanothieno[3,2-b]thiophene, belongs to the family of fused thiophenes which are electron-rich and structurally rigid with extended π-conjugation. They are good candidates for adjusting the band gap of organic polymer semiconducting materials. Thieno[3,2-b]thiophene-2-carbonitrile is used as an intermediate for the synthesis of small molecules and polymers in the application of organic field-effect transistors (OFETs), and as organic photovoltaic devices (OPV) interface layers as HTL or ETL materials.
![Synthesis of 3,6-bis(thieno[3,2-b]thiophen-5-yl)-2,5-bispyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione with Thieno[3,2-b]thiophene-2-carbonitrile as starting material](https://www.ossila.com/cdn/shop/files/synthesis-DTT-DPP.jpg?v=1718792508)
Bearing a nitrile group
For a facial synthesis of pyrrolopyrrole
Thienothiophene building block
For semiconductors, OFETs, and solar cells
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 40985-58-8 |
| Chemical Formula | C7H3NS2 |
| Molecular Weight | 165.23 g/mol |
| Synonyms | 2-Cyanothieno[3,2-b]thiophene |
| Classification / Family | Thiophene, Thienothiophene, Fused thiophene, Heterocyclic five-membered ring, Organic materials, Semiconductor synthesis, Low band gap polymers, OFETs, Organic photovoltaics, Polymer solar cells |
Chemical Structure
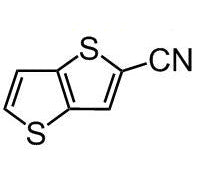
Product Details
| Purity | 98% |
| Melting Point | 48 °C - 50 °C |
| Appearance | Off-white powder/crystals |
Literature and Reviews
- Thieno[3,2-b]thiophene-diketopyrrolopyrrole-containing polymers for high-performance organic field-effect transistors and organic photovoltaic devices, H. Bronstein et al., J. Am. Chem. Soc., 133 (10), 3272–3275 (2011), DOI: 10.1021/ja110619k.
- Alkyl Chain Extension as a Route to Novel Thieno[3,2-b]thiophene Flanked Diketopyrrolopyrrole Polymers for Use in Organic Solar Cells and Field Effect Transistors, I. Meager et al., Macromolecules, 46 (15), 5961–5967 (2013), DOI: 10.1021/ma401128s.
- Photocurrent Enhancement from Diketopyrrolopyrrole Polymer Solar Cells through Alkyl-Chain Branching Point Manipulation, I. Meager et al.,, J. Am. Chem. Soc., 135 (31), 11537–11540 ( 2013 ), DOI: 10.1021/ja406934j.
![Thieno[3,2-b]thiophene-2-carbonitrile CAS 40985-58-8](http://www.ossila.com/cdn/shop/files/thienothiophene-2-carbonitrile.jpg?v=1718725228&width=380)