5,5'-bis(trimethylstannyl)-2,2'-bithiophene
CAS Number 143367-56-0
Chemistry Building Blocks, Heterocyclic Building Blocks, Materials, Monomers,High purity monomer for applications in OFETs, OLED, PLED and OPV
For the synthesis of small molecules and semiconducting polymers
Specifications | MSDS | Literature and Reviews
5,5'-bis(trimethylstannyl)-2,2'-bithiophene, CAS number 143367-56-0, can be used for the synthesis of small molecules and polymer semiconductors for OFETs, OLED, PLED, OPV applications.5,5'-bis(trimethylstannyl)-2,2'-bithiophene can be prepared from 2,2'-bithiophene which is an electron rich and donating unit if it is embedded into low band-gap polymer semiconductors.
Reacting with 4,9-dibromo-2,7-bis(2-octyldodecyl)-Benzo[3,8]phenanthroline-1,3,6,8-(2H,7H)-tetrone, 5,5'-bis(trimethylstannyl)-2,2'-bithiophene yields a heavily n-dopable π-conjugated redox polymer, poly{[N,N′-bis(2-octyldodecyl)-1,4,5,8-naphthalenedicarboximide-2,6-diyl]-alt-5,5′-(2,2′-bithiophene)} (PNDI2OD-T2).
![Synthesis of PNDI2OD-T2 by reacting 4,9-dibromo-2,7-bis(2-octyldodecyl)-Benzo[3,8]phenanthroline-1,3,6,8(2H,7H)-tetrone with 5,5'-bis(trimethylstannyl)-2,2'-bithiophene via Stille Coupling polymerisation](https://www.ossila.com/cdn/shop/files/PNDI2OD-T2-synthesis.jpg?v=1718808166)
Note: This product has been used in our own lab by for the synthesis of PDPP4T and DPPDPyBT (n-type).
Bithiophene building block
for semiconductors, OFETs, and solar cells
Worldwide shipping
Quick and reliable shipping
Capped with trimethyltin
a monomer for PDPP4T and DPPDPyBT
High purity
>98% Purity
General Information
| CAS Number | 143367-56-0 |
| Chemical Formula | C14H22S2Sn2 |
| Molecular Weight | 491.87 g/mol |
| Synonyms | 5,5-Ditrimethylstannyl-2,2'-bithiophene 5,5'-Bis(trimethyltin)-2,2'-bithiophene |
| Classification / Family | Bithiophene, Thiophene, Heterocyclic five-membered ring, Organic semiconducting materials, Semiconductor Synthesis, Low band gap polymers, OFETs, OLED, Organic Photovoltaics, Polymer Solar Cells |
Chemical Structure
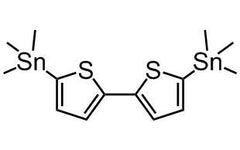
Product Details
| Purity | >98% |
| Melting Point | 96 °C - 100 °C |
| Appearance | White flakes |
Characterization
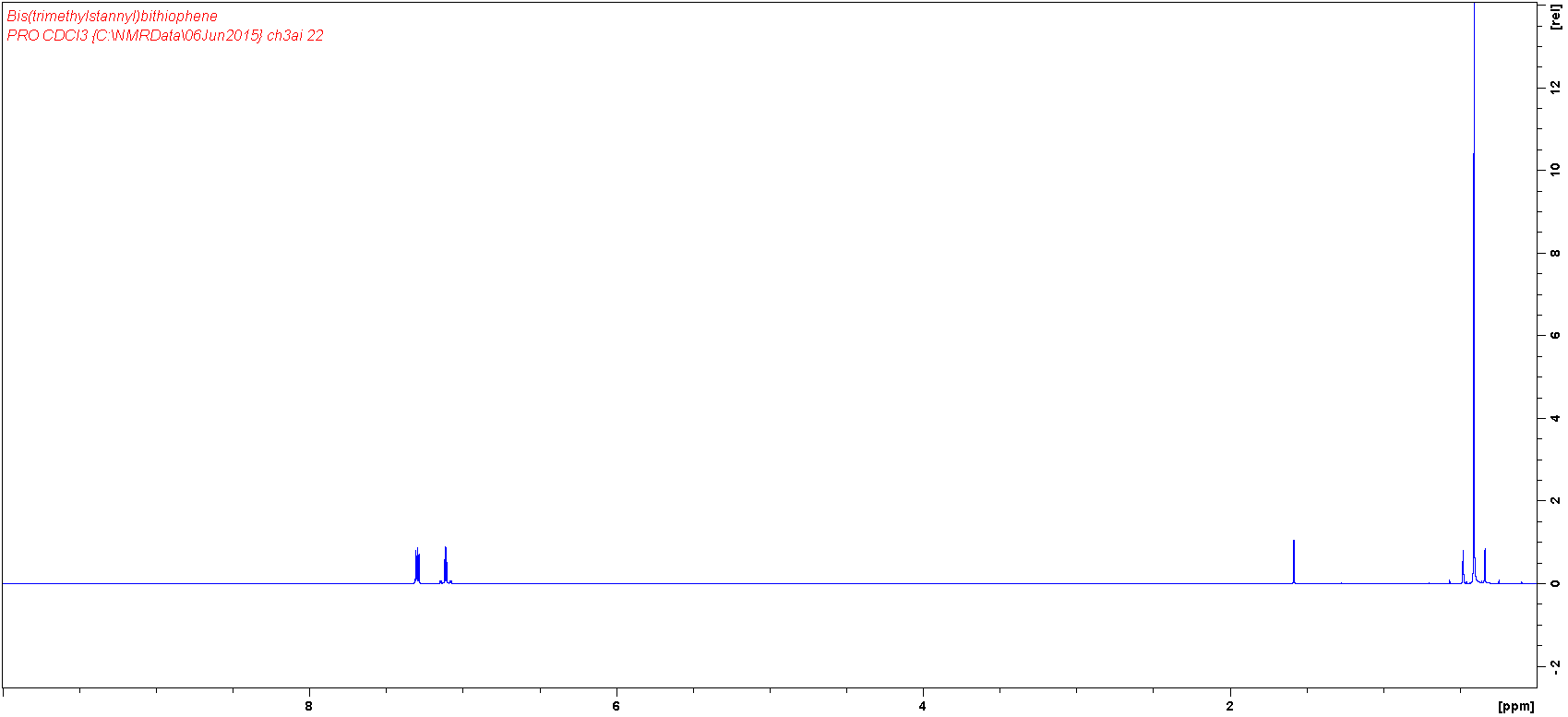
MSDS Documentation
5,5'-bis(trimethylstannyl)-2,2'-bithiophene MSDS sheet
Literature and Reviews
- A Polythiophene Derivative with Superior Properties for Application in Polymer Solar Cells, M. Zhang, Adv. Mater., 26 (33), 5880-5885 (2014)
- Low Band-Gap Conjugated Polymers with Strong Interchain Aggregation and Very High Hole Mobility Towards Highly Efficient Thick-Film Polymer Solar Cells, Z. Chen, Adv. Mater., 26 (16), 2586-2591 (2014)
- A high-mobility electron-transporting polymer for printed transistors, H. Yan, Nat. Mater., 457, 679 (2009)
