2,2'-bithiophene
CAS Number 492-97-7
Chemistry Building Blocks, Heterocyclic Building Blocks, Materials, MonomersHigh purity 2,2'-bithiophene, for applications in organic electronics
For the synthesis of small molecules or polymer semiconductors
Specifications | MSDS | Literature and Reviews
2,2'-bithiophene (CAS number 492-97-7) is an intermediate widely used for the synthesis of small molecules or polymer semiconductors in application of organic electronics. It has proven that 2,2'-bithiophene exists as a mixture of the cis-like and the trans-like planar structures. 5,5'-positions of 2,2'-bithiophene are easily accessible for bromination and stannylation to give 5,5'-dibromo-2,2'-bithiophene or 5,5'-trimethylstannyl-2,2'-bithiophene, which can be used for direct arylation reactions.

A synthesis precusor
For bithiophene backboned polymers
Bithiophene building block
For semiconductors, OFETs, and solar cells
Worldwide shipping
Quick and reliable shipping
High purity
>99% High purity
General Information
| CAS Number | 492-97-7 |
| Chemical Formula | C8H6S2 |
| Molecular Weight | 166.26 g/mol |
| Synonyms | 2,2′-Bithienyl, 2,2′-Dithienyl 2-(2-thienyl)thiophene 2-(thien-2-yl)thiophene |
| Classification / Family | Thiophene, Bithiophene, Heterocyclic five-membered ring, Organic materials, Semiconductor Synthesis, Low band gap polymers, OFETs, Organic Photovoltaics, Polymer Solar Cells |
Chemical Structure
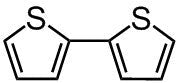
Product Details
| Purity | 99% |
| Melting Point | 32 °C - 33 °C |
| Appearance | Yellowish liquid/solid |
Characterization by 1H-NMR (example)
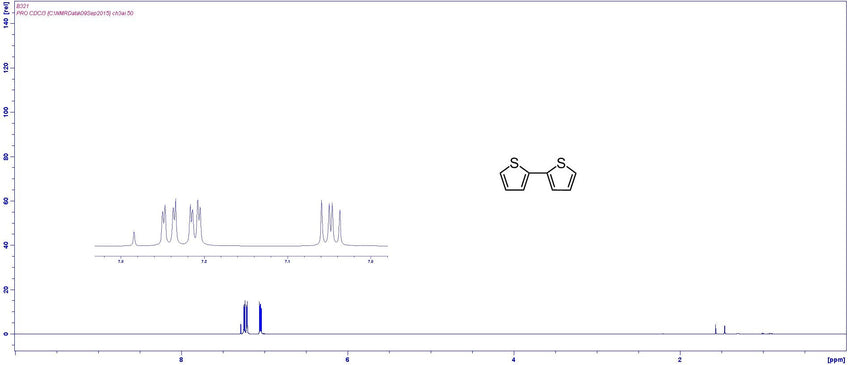
MSDS Documentation
Literature and Reviews
- White light from an electroluminescent diode made from poly[3(4‐octylphenyl)‐2,2’‐bithiophene] and an oxadiazole derivative, M. Berggren et al, J. Appl. Phys. 76, 7530 (1994); http://dx.doi.org/10.1063/1.357984.
- Electronic structure of thiophene and pyrrole dimers: 2,2’‐bithiophene, 2,2’‐thienylpyrrole, and 2,2’‐bipyrrole, D. Birnbaum et al., J. Chem. Phys. 95, 4783 (1991); http://dx.doi.org/10.1063/1.461721.
- Highly Efficient Light-Harvesting Ruthenium Sensitizer for Thin-Film Dye-Sensitized Solar Cells, C-Y. Chen et al., ACS Nano, 3 (10), 3103–3109 (2009); DOI: 10.1021/nn900756s.
