PDPP4T
CAS Number 1267540-03-3
Chemistry Building Blocks, Interface Polymers, Luminosyn™ Polymers, Materials,Low Band-Gap Semiconducting Polymer for Organic Solar Cells
High purity polymer available for fast and secure dispatch
Specifications | MSDS | Literature and Reviews | Technical Support
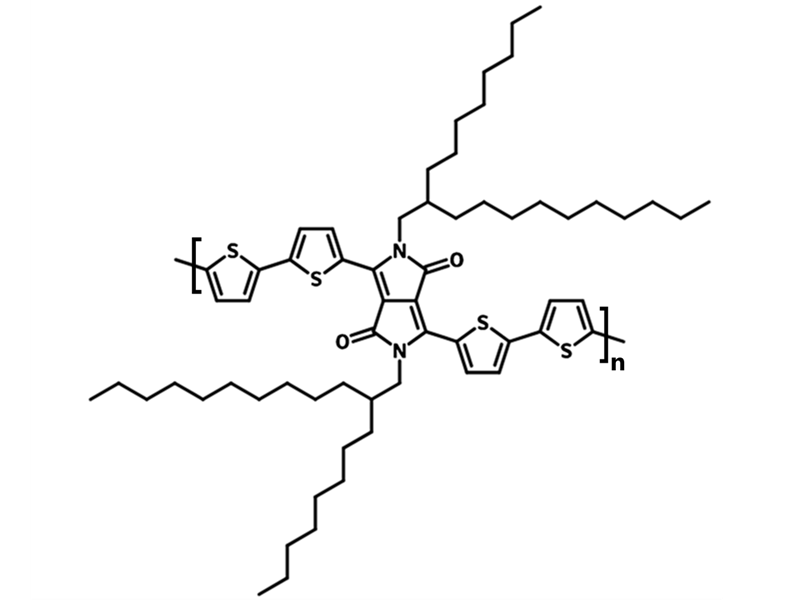
PDPP4T (CAS number 1267540-03-3) is a promising class of semiconducting polymers for organic solar cells. This is due to its small optical band gap and high charge-carrier mobility. It is also known as Poly[2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione -3,6-diyl)-alt-(2,2’;5’,2’’;5’’,2’’’-quaterthiophen-5,5’’’-diyl)] or DPP4T.
PDPP4T from Ossila was used in the high-impact paper (IF 14.92), Tuning charge transport dynamics via clustering of doping in organic semiconductor thin films, C. Boyle et al., Nat. Commun., 10, 2827 (2019); DOI: 10.1038/s41467-019-10567-5.
With one DPP unit as electron-withdrawing and four five-membered thiophene as electron-rich units in its backbone, PDPP4T is a low-band gap polymer semiconductor with a planar structure. The alkyl chain attached to the DPP unit serves as a high-solubilizing group and has a tendency to crystallize to ensure a better packing film. A hole mobility greater than 1 cm2 V-1 s-1 [8] has been reported in top-contact bottom-gate devices.
Device performance PCE of 7.59% with a VOC of 0.61 V, JSC of 17.95 mA/cm 2 , and FF of 69.6%, is reported with PC71BM as electron acceptor [1]. This is using a solvent swelling assisted sequential deposition (SSA-SD) method to produce bulk heterojunction PSCs based on a crystalline diketopyrrolopyrrole (DPP) polymer and PC71BM. By adding polymers with high mobility, like DPP-DTT, device performance with higher PCE should be expected [5].
Please enquire for 5-10 grams order quantity, the lead time is 4-6 weeks.
The Luminosyn™ Range
General Information
| Full Name | Poly[2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione -3,6-diyl)-alt-(2,2’;5’,2’’;5’’,2’’’-quaterthiophen-5,5’’’-diyl)] |
| Synonyms |
|
| CAS Number | 1267540-03-3 |
| Chemical Formula | (C62H90N2O2S4)n |
| HOMO and LUMO | HOMO = -5.2 eV, LUMO = -4.0 eV [7] |
| Soluble in | Chloroform |
| Recommended Processing Solvents at 10mg/ml | Chloroform |
| Classification or Family | Quaterthiophene, Heterocyclic five-membered ring, Organic semiconducting materials, Low band gap polymers, Organic photovoltaics, Polymer solar cells, OFETs |
Batch Details
| Batch number | MW | Mn | PDI | Stock info |
|---|---|---|---|---|
| M0331A3 | 61,447 | 36,310 | 1.69 | In stock |
Chemical Structure
Characterization
Soxhlet extraction was carried out using methanol, acetone, hexane, and then chlorobenzene as washing solvents under argon. Chlorobenzene fraction was concentrated, precipitated with methanol, and dried under vacuum at 40 oC for 48 hours. GPC was carried out using 1,2,4-trichlorobenzene as eluent at 140 oC by using polystyrene as standards.
Synthetic Route
DPP4T was synthesized by using 3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione and 5,5'-bis(trimethylstannyl)-2,2'-bithiophene as starting materials via Stille Coupling polymerization in chlorobenzene. Targeted polymer was purified using Soxhlet extraction with methanol, acetone, hexane and finally chlorobenzene as washing and extracting solvents.
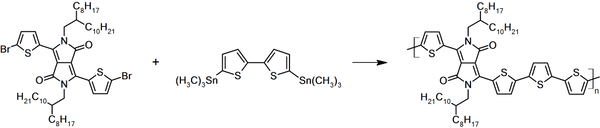
MSDS Documentation
Literature and Reviews
- Sequential Deposition: Optimization of Solvent Swelling for High-Performance Polymer Solar Cells, Y. Liu et al., ACS Appl. Mater. Interfaces, 7, 653-661 (2015)
- Copolymers of diketopyrrolopyrrole and thienothiophene for photovoltaic cells, J.C. Bijleveld et al., J. Mater. Chem., 21, 9224-9231 (2011)
- Diketopyrrolopyrrole-Based π‑Conjugated Copolymer Containing β‑Unsubstituted Quintetthiophene Unit: A Promising Material Exhibiting High Hole-Mobility for Organic Thin-Film Transistors, Z. Yi et al., Chem. Mater., 24, 4350-4356 (2012)
