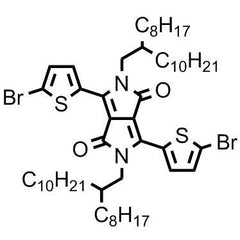
3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione
CAS Number 1260685-63-9
Chemistry Building Blocks, Heterocyclic Building Blocks, Materials, MonomersHigh purity monomer, used to optimize the performance of organic semiconductors
Free worldwide shipping on qualifying orders
Specifications | MSDS | Literature and Reviews
The monomer, 3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione, CAS Number 1260685-63-9, contains a DPP moiety. DPP backbone derivatives as a strong acceptor unit, have a planar conjugated bicyclic structure engaging stronger π–π interactions which allows tuning the band-gap and energy-level of polymer organic semiconductors. Also long alkyl chains, namely 2-octyldodecyl, will allow better solubility for the polymer semiconductor and crystallization and aggregation capacity for the thin-film devices. DPP-containing polymers are considered promising materials for optimizing the performance of organic semiconductors.
2,5-Bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4-(2H,5H)-dione-based donor-acceptor alternating copolymer bearing 5,5'-di(thiophen-2-yl)-2,2'-biselenophene exhibiting 1.5 cm2/Vs hole mobility in thin-film transistors.
![pdpp-alt-dtbse-synthesis with 3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione and 5,5’-di(thiophen-2-yl)-2,2’-biselenophene](https://www.ossila.com/cdn/shop/files/PDPP-alt-DTBSe-synthesis.jpg?v=latest)
Note: This product has been used in our own lab by Ossila chemist for the synthesis of PDPP4T and high mobility p-type polymer DPP-DTT.
Capped with bromide
A DPP-DTT monomer
Pyrrolopyrrole building block
For semiconductors, OFETs, and solar cells
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 1260685-63-9 |
| Chemical Formula | C54H86Br2N2O2S2 |
| Molecular Weight | 1019.2 g/mol |
| Synonyms |
|
| Classification / Family | Pyrroles, Thiophenes, Acceptor, Organic semiconducting materials, Semiconductor synthesis, Building blocks |
Chemical Structure
Product Details
| Purity | >98% |
| Melting Point | 95 °C - 97 °C |
| Appearance | Dark purple solid |
NMR Characterization
![1H NMR 3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione](https://www.ossila.com/cdn/shop/files/nmr-dibromothienyl-octyldodecyl-dpp.png?width=848&height=433)
MSDS Documentation
Literature and Reviews
- Effect of Selenophene in a DPP Copolymer Incorporating a Vinyl Group for High-Performance Organic Field-Effect Transistors, I. Kang et al., Adv. Mater., 25, 524-528 (2013).
- Synthesis, Characterization, and Field-Effect Transistors Properties of Novel Copolymers Incorporating Nonplanar Biindeno[2,1-b]thiophenylidene Building Blocks, C. Li, Macromolecules, 48 (8), 2444-2453 (2015).
![3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione CAS 1260685-63-9](http://www.ossila.com/cdn/shop/files/bisbromothiophenyl-bisoctyldodecylpyrrolo-dione.jpg?v=1718718829&width=380)