IDTB6-2Sn
CAS Number 1252555-61-5
Chemistry Building Blocks, Heterocyclic Building Blocks, Materials, Monomers, Organotin CompoundsIDTB6-2Sn, high purity monomer
An intermediate in polymer organic solar cells for low band gap polymers and NFAs
Specifications | MSDS | Literature and Reviews
4,4,9,9-tetrakis(4-hexylphenyl)-4,9-dihydro-s-indaceno[1,2-b:5,6-b']dithiophene-2,7-diyl)bis(trimethylstannane) (IDTB6-2Sn), CAS number 1252555-61-5, has a structure of four hexylphenyl groups attached to an indaceno[1,2-b:5,6-b']dithiophene (IDT) core. IDTB6-2Sn has trimethylstannyl end functional groups for Stille coupling reaction to further extend its conjugation and enable absorption into the near-infrared region.
In polymer organic solar cells, IDTB6-2Sn is used as an intermediate for more complicated structures such as low bandgap polymers or non-fullerene acceptors, such as IDT-2Br or IEICO-4F.
Part of our range of non-fullerene acceptor monomers, we stock high purity (>98%) IDTB6-2Sn for priority dispatch (lead times may apply for large quantities, please contact us for details). Qualifying orders ship free.
Enabling facile coupling reaction
With trimethylstannyl end groups
High purity
>98% IDTB6-2Sn Purity
Worldwide shipping
Quick and reliable shipping
Electron-rich building block
Preparation of low bandgap non-fullerene acceptors (NFAs)
General Information
| CAS Number | 1252555-61-5 |
| Chemical Formula | C70H90S2Sn2 |
| Molecular Weight | 1233.03 g/mol |
| Full Name | 4,4,9,9-tetrakis(4-hexylphenyl)-4,9-dihydro-s-indaceno[1,2-b:5,6-b']dithiophene-2,7-diyl)bis(trimethylstannane) |
| Synonyms |
IDT-nC6 1,1'-[4,4,9,9-tetrakis(4-hexylphenyl)-4,9-dihydro-s-indaceno[1,2-b:5,6-b']dithiophene-2,7-diyl]bis[1,1,1-trimehtylstannyl]stannane |
| Classification / Family | Indaceno[1,2-b:5,6-b']dithiophene (IDT), Monomer and intermediates, None-fullerene acceptors (NFAs), NFA-OSCs, printing electronics |
Chemical Structure
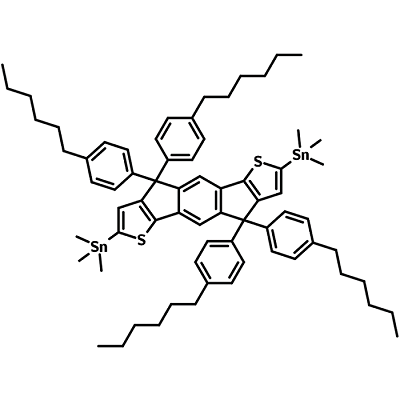
Product Details
| Purity | >98% (by NMR) |
| Melting Point | N/A |
| Appearance | Yellow crystalline powder |
MSDS Documentation
Literature and Reviews
- Indacenodithiophene-Based Wide Bandgap Copolymers for High Performance Single-Junction and Tandem Polymer Solar Cells. Y.Ma et al., Nano Energy, 33, 313-324 (2017); DOI: 10.1016/j.nanoen.2017.01.050.
- A cyclopentadithiophene-bridged small molecule acceptor with near-infrared light absorption for efficient organic solar cells, Y. Yi et al, J. Mater. Chem. C, 7, 4013-4019 (2019); DOI: 10.1039/c9tc00162j.
- Efficient Semitransparent Solar Cells with High NIR Responsiveness Enabled by a Small-Bandgap Electron Acceptor, F. Liu et al., 29 (21), 1606574 (2017); DOI: 10.1002/adma.201606574.
