Benzodithiophene-2THBr-4EH
CAS Number 1439937-07-1
Chemistry Building Blocks, Dibromo Monomers, Heterocyclic Building Blocks, Materials,Benzodithiophene-2THBr-4EH, high purity monomer for applications in OPVs and OFETs
For the synthesis of low band-gap polymer semiconductors
Specifications | MSDS | Literature and Reviews
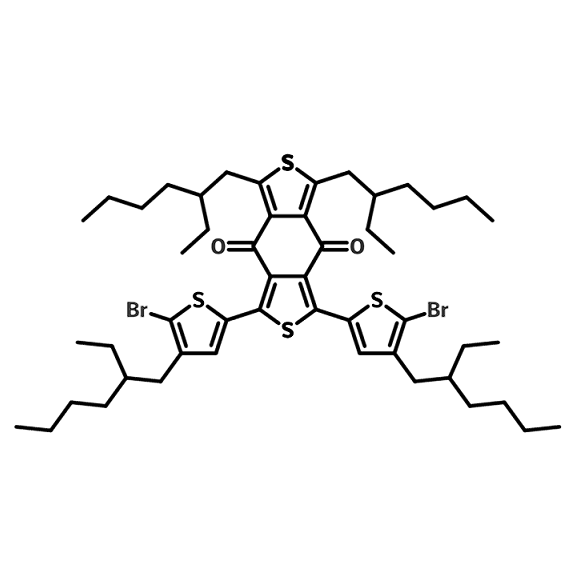
1,3-Bis(5-bromo-4-(2-ethylhexyl)thiophen-2-yl)-5,7-bis(2-ethylhexyl)benzo[1,2-c:4,5-c']dithiophene-4,8-dione, or Benzodithiophene-2THBr-4EH, is a monomer for the synthesis of low band-gap polymer semiconductors such as PBDD4T and PBDD4T-2F. It has applications in OPV (fullerene or fullerene-free), tandem organic solar cells, and OFETs.
Benzodithiophene-2THBr-4EH has a more soluble structure due to its four branched side chains, so resulting polymers can be more easily processed for device fabrications.
General Information
| CAS Number | 1439937-07-1 |
| Chemical Formula | C50H70Br2O2S4 |
| Molecular Weight | 991.16 g/mol |
| Synonyms | 1,3-Bis(5-bromo-4-(2-ethylhexyl)thiophen-2-yl)-5,7-bis(2-ethylhexyl)benzo[1,2-c:4,5-c']dithiophene-4,8-dione |
| Classification / Family | Thiophene, Fused thiophene, Benzodithiophene dione, Semiconductor synthesis intermediates, Low band gap polymers, OFETs, Organic photovoltaics, Polymer solar cells |
Chemical Structure
Product Details
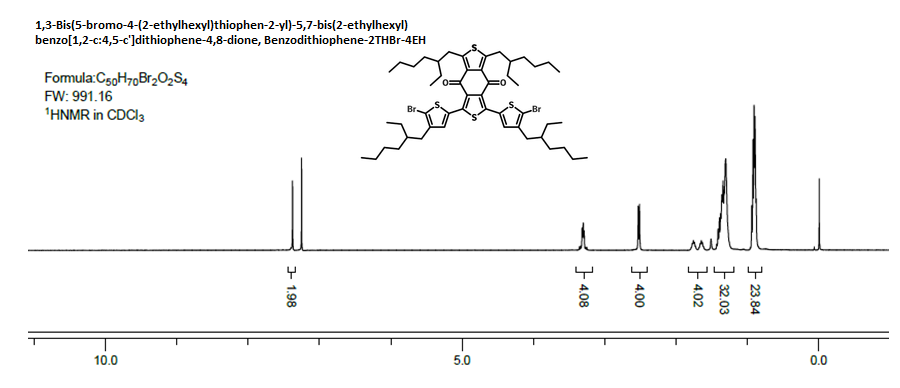
| Purity | >98% (by 1H-NMR in CDCl3) |
| Melting Point | n/a |
| Appearance | Yellowish-orange powder/crystals |
Characterization
MSDS Documentation
Benzodithiophene-2THBr-4EH MSDS sheet
Literature and Reviews
- A Fluorinated Polythiophene Derivative with Stabilized Backbone Conformation for Highly Efficient Fullerene and Non-Fullerene Polymer Solar Cells, S. Zhang et al., Macromolecules, 49 (8), 2993–3000 (2016); DOI: 10.1021/acs.macromol.6b00248.
- Achieving 12.8% Efficiency by Simultaneously Improving Open-Circuit Voltage and Short-Circuit Current Density in Tandem Organic Solar Cells, Y. Qin et al., Adv. Mater. 2017, 29, 1606340 (2017); DOI: 10.1002/adma.201606340.
