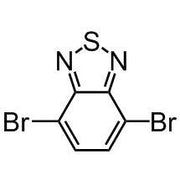
4,7-Dibromo-2,1,3-benzothiadiazole
CAS Number 15155-41-6
Chemistry Building Blocks, Dibromo Monomers, Heterocyclic Building Blocks, Materials, MonomersHigh purity monomer for organic semiconductor synthesis
Used for applications in LEDs and photovoltaic devices
Specifications | MSDS | Literature and Reviews
4,7-Dibromo-2,1,3-benzothiadiazole, CAS number 15155-41-6, is the building block or monomer for organic semiconductors synthesis in the application of light-emitting diodes and photovoltaic devices. Intermediate for the synthesis of i.e. PCDTBT and PCPDTBT.
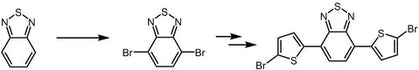
4,7-Dibromo-2,1,3-benzothiadiazole can be synthesized by using 2,1,3-benzothiadiazole as starting materials to react with bromine in hydrobromic acid. Further bromination of 4,7-Dibromo-2,1,3-benzothiadiazole affords 4,7-bis(5-bromothiophen-2-yl)benzo[c][1,2,5]thiadiazole, a red monomer leading to our pilot polymer PCDTBT which can be used for OPV devices. 4,7-Dibromo-2,1,3-benzothiadiazole is also used for the synthesis of PCPDTBT, a good candidate for the polymer solar cell devices.
Benzothiadiazole building block
for semiconductors, OFETs, and solar cells
Worldwide shipping
Quick and reliable shipping
Capped with bromide
a PCDTBT monomer
High purity
>98% Purity
General Information
| CAS Number | 15155-41-6 |
| Chemical Formula | C6H2Br2N2S |
| Molecular Weight | 293.97 g/mol |
| Synonyms | Dibromo Benzothiodiazole Dibromo-BT |
| Classification / Family | Benzothiodiazole, PCPDTBT, Semiconductor synthesis, Low band gap polymers, Acceptors, Organic Photovoltaics, Polymer solar cells |
Chemical Structure
Product Details
| Purity | ≥98% |
| Melting Point | 187 °C - 190 °C |
| Appearance | Light yellow crystals |
Characterization
MSDS Documentation
4,7-Dibromo-2,1,3-benzothiadiazole MSDS sheet
Literature and Reviews
- Introduction of Perylene Units for Enhanced Interchain Interaction in Conjugated Polymers for Organic Photovoltaic Devices, K. Ji-Hoon et al., Macromolecules, 45(5), 2367-2376 (2012)
- Fluorinated Copolymer PCPDTBT with Enhanced Open-Circuit Voltage and Reduced Recombination for Highly Efficient Polymer Solar Cells, S. Albrecht et al., J. Am. Chem. Soc., 134, 14932-14944 (2012)
