4H-Cyclopenta[1,2-b:5,4-b']dithiophene
CAS Number 389-58-2
Chemistry Building Blocks, Heterocyclic Building Blocks, Materials, MonomersHigh purity CPDT monomer used in OFETs and organic electronics
Available online for fast, secure dispatch
Specifications | MSDS | Literature and Reviews
4H-cyclopenta[2,1-b:3,4-b′]dithiophene, also known as CPDT (CAS number 389-58-2), a rigid coplanar structure favoring π−π intermolecular interactions with good electron-donating properties, has been one of the most attractive building blocks for organic field effect transistors and organic electronics. The five-member ring in the middle also offer the function to have side-chain manipulation to enhance solubility in solutions for device fabrications, morphology and polymer processing. One of the intensively studied polymers for organic electronics, PCPDTBT, with the alternating CPDT and 2,1,3- benzothiadiazole (BT) units, has demonstrated device performance of PCE over 6% [5]
Recently, the CPDT backboned polymer, CPE-K, has been incorporated as the electron hole transporting layer (HTL) in the polymer/fullerene BHJ and perovskite solar cells. The devices using CPE-K HTL show competitive performance compared with that using PEDOT:PSS HTL. As an alternative to PEDOT:PSS, the use of CPE-K as an organic hole transport material enables the formation of uniform perovskite films with complete surface coverage for an efficient, stable perovskite/fullerene planar heterojunction solar cell [1, 2]
4H-cyclopenta[2,1-b:3,4-b′]dithiophene is the intermediate for the synthesis of 4,4'-alkyl-cyclopenta[2,1-b:3,4-b′]dithiophene [6].
![synthesis of 4,4'-alkyl-cyclopenta[2,1-b:3,4-b′]dithiophene](https://www.ossila.com/cdn/shop/files/alkylted-cyclopentadithiophene-synthesis.jpg?v=1718809140)
A synthesis precusor
For cyclopentadithiophene backboned polymers
Cyclopentadithiophene building block
For semiconductors, OFETs, and solar cells
Worldwide shipping
Quick and reliable shipping
High purity
>99% High purity
General Information
| CAS Number |
389-58-2 |
| Chemical Formula | C9H6S2 |
| Molecular Weight | 178.27 g/mol |
| Synonym | 4H-Cyclopenta[2,1-b:3,4-b']dithiophene 4H-Thieno[3',2':4,5]cyclopenta[1,2-b]thiophene 3,4-Dithia-7H-cyclopenta[a]pentalene CPDT |
| Classification / Family | Monomers, Building blocks, Heterocycles, Chemical synthesis for low-band gap polymers, Thiophene, Intermediates for high performance Organic Photovoltaics, OFETs, Polymer solar cells |
Chemical Structure
![Chemical structure of 4H-Cyclopenta[2,1-b:3,4-b']dithiophene CAS 389-58-2](https://www.ossila.com/cdn/shop/files/cyclopentadithiophene.jpg?v=1726134723)
Product Details
| Purity | >99% |
| Melting Point | 71.0 °C - 75.0 °C |
| Appearance | Off-white powder |
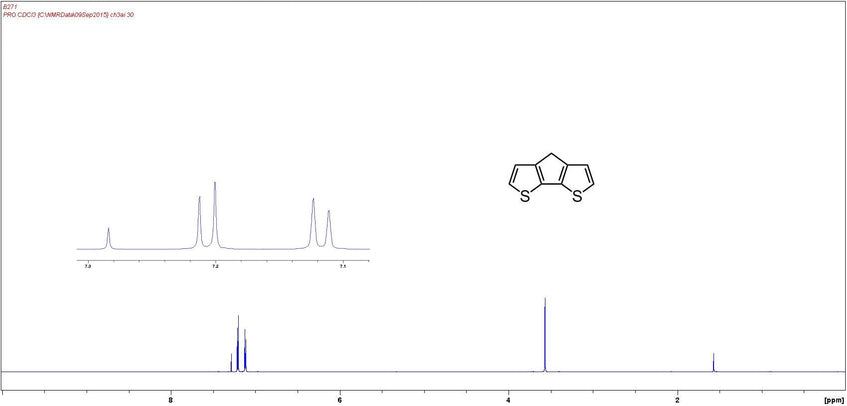
NMR Characterization
MSDS Documentation
4H-Cyclopenta[1,2-b:5,4-b']dithiophene MSDS sheet
Literature and Reviews
- Conjugated polyelectrolyte hole transport layer for inverted-type perovskite solar cells, H. Choi et al., Nat. Commun., DOI: 10.1038/ncomms8348, 6, 7348 (2015)
- Conductive conjugated polyelectrolyte as hole-transporting layer for organic bulk heterojunction solar cells, H. Zhou, et al., Adv. Mater. , 26, 780–785(2014)
- Improved Photovoltaic Performance of a Semicrystalline Narrow Bandgap Copolymer Based on 4H-Cyclopenta[2,1-b:3,4- b′]dithiophene Donor and Thiazolo[5,4-d]thiazole Acceptor Units, S. V. Mierloo et al., Chem. Mater., 24, 587−593 (2012).
![4H-Cyclopenta[1,2-b:5,4-b']dithiophene CAS 389-58-2](http://www.ossila.com/cdn/shop/files/cyclopentadithiophene.jpg?v=1726134723&width=380)