2,2'-bithiophene-5-carbonitrile
CAS Number 16278-99-2
Chemistry Building Blocks, Heterocyclic Building Blocks, Materials, Monomers2,2'-bithiophene-5-carbonitrile, high-purity monomer
For the synthesis of semiconducting materials used in organic electronic devices
Specifications | MSDS | Literature and Reviews
2,2'-bithiophene-5-carbonitrile (CAS number 16278-99-2) is the intermediate used for the synthesis of 3,6-bis[5-(2,2'-bithiophene)]pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione which can be further modified to form oligomers or polymers used for organic electronic devices.
2,2'-bithiophene unit is electron-rich, and thus a good building block for the synthesis of semiconducting materials that are used for organic electronic devices. Embedded in the polymer backbones, 2,2'-bithiophene unit is proven to promote cystallinity to form small domains in bulk heterojunction solar cells [4].
![Synthesis of 3,6-bis[5-(2,2'-bithiophene)]pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione](https://www.ossila.com/cdn/shop/files/synthesis-DBT-DPP.jpg?width=480&height=116)
Bithiophene building block
For the synthesis of OLED and organic photovoltaic materials
Worldwide shipping
Quick and reliable shipping
Functionlized with a nitrile group
For synthesizing pyrrolopyrrole derivatives
High purity
>98% Purity
General Information
| CAS Number | 16278-99-2 |
| Chemical Formula | C9H5NS2 |
| Molecular Weight | 191.27 g/mol |
| Synonyms |
|
| Classification / Family | Thiophene, Bithiophene, Heterocyclic five-membered ring, Organic materials, Semiconductor synthesis, Building blocks |
Chemical Structure
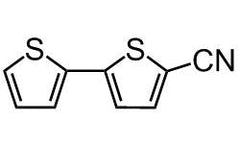
Product Details
| Purity | >98% |
| Melting Point | 74 °C - 75 °C |
| Appearance | White powder/crystals |
NMR Characterization
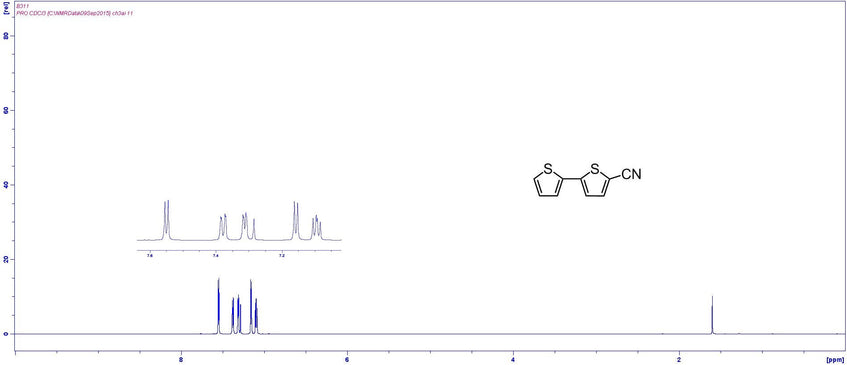
MSDS Documentation
2,2'-bithiophene-5-carbonitrile MSDS sheet
Literature and Reviews
- Effect of substituents on redox, spectroscopic and structural properties of conjugated diaryltetrazines—a combined experimental and theoretical study, E. Kurach et al., Phys. Chem. Chem. Phys., 13, 2690–2700 (2011).
- Dicationic phenyl-2,2′-bichalcophenes and analogs as antiprotozoal agents, M. A. Ismail et al., Bioorg. Med. Chem. 19, 978–984 (2011).
- Compromise between conjugation length and charge-transfer in nonlinear optical h5 -monocyclopentadienyliron(II) complexes with substituted oligo-thiophene nitrile ligands: Synthesis, electrochemical studies and first hyperpolarizabilities, M. H. Garcia et al., J. Organometallic Chem., 692, 3027–3041 (2007).
