BT-C12-2Br
CAS Number 753470-95-0
Chemistry Building Blocks, Dibromo Monomers, Heterocyclic Building Blocks, Materials,Bithiophene derivate, BT-C12-2Br
Commonly used for the synthesis of polymer semiconductors PBTTT-C12 and PQT12
Specifications | MSDS | Literature and Reviews
BT-C12-2Br, or 5,5′-Dibromo-4,4′-didodecyl-2,2′-bithiophene (CAS number 753470-95-0), is a bithiophene derivate with unbranched dodecyl alkyl (C12) side chains. Dodecyl alkyl chains can not only enhance solubility of the targeted semiconducting polymers, but also promote film morphology with better intermolecular packing and π–π stacking.
BT-C12-2Br has been widely used for the synthesis of polymer semiconductors PBTTT-C12 and PQT12. These can be used to create organic field-effect transistors (OFETs) with high charge mobilities.
We stock high purity (>98%) BT-C12-2Br as part of our range of monomers. Order online or request a quote today.
Bithiophene building block
for the synthesis of OLED and organic photovoltaic materials
Worldwide shipping
Quick and reliable shipping
Capped with bromides
for facile coupling reactions
High purity
>98% Purity
General Information
| CAS Number | 753470-95-0 |
| Chemical Formula | C32H52Br2S2 |
| Full Name | 5,5′-Dibromo-4,4′-didodecyl-2,2′-bithiophene |
| Molecular Weight | 660.69 g/mol |
| Synonyms | 2,2'-dibromo-3,3'-bis(n-dodecyl)-5,5'-bithiophene |
| Classification / Family | Bithiophene, semiconductor synthesis intermediates, low band gap polymers, OFETs, organic photovoltaics, polymer solar cells |
Chemical Structure
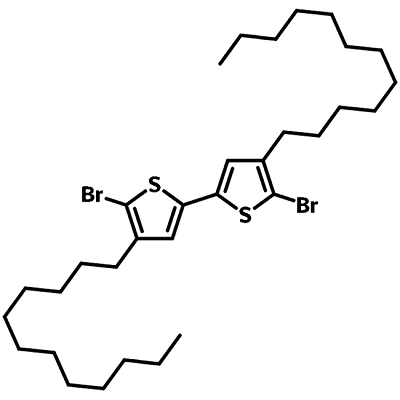
Product Details
| Purity | >98% (1H NMR in CDCl3) |
| Melting Point | 56 °C - 60 °C |
| Appearance | Yellow flakey solid |
MSDS Documentation
Literature and Reviews
- High-Performance Semiconducting Polythiophenes for Organic Thin-Film Transistors, B. Ong et al., J. Am. Chem. Soc., 126, 11, 3378–3379 (2004); DOI: 10.1021/ja039772w.
- Factors Governing Intercalation of Fullerenes and Other Small Molecules Between the Side Chains of Semiconducting Polymers Used in Solar Cells, N. Miller et al., Adv. Energy. Mater., 2 (10), 1208-1217 (2012); DOI: 10.1002/aenm.201200392.
- Remarkable Suppression of Vibrational Relaxation in Organic Semiconducting Polymers by Introducing a Weak Electron Donor for Improved NIR-II Phototheranostics, C. Yin et al., Adv. Funct. Mater., 31 (47), 2106575 (2021); DOI: 10.1002/adfm.202106575.
