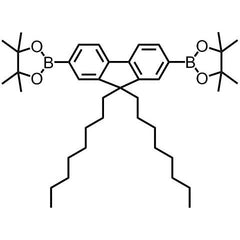
2,2'-(9,9-dioctyl-9H-fluorene-2,7-diyl)bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)
CAS Number 196207-58-6
Boronates, Chemistry Building Blocks, Materials, Monomers,High quality precursor for the synthesis of semiconducting polymers
High purity monomer available online for fast, secure dispatch
Specifications | MSDS | Literature and Reviews
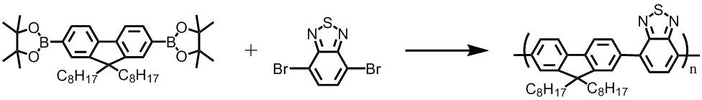
2,2'-(9,9-dioctyl-9H-fluorene-2,7-diyl)bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane), CAS number 196207-58-6, is a precursor for the synthesis of polymer semiconductors in the application of OLED, PLED, OFET and Polymer Solar Cells.
With their pure blue and efficient electroluminescence, high charge-carrier mobility and good processability, alkylated polyfluorene conjugated polymer semiconducting materials have attracted great research and industrial interests -- especially for display technology photovoltaic applications. PFN, a fluorene back-boned polymer semiconductor, is a conjugated polyelectrolyte embedded between the cathode and the active layer in OPV devices to improve electron extraction efficiencies. Both PFO (F8) and F8BT use the same polyfluorene sub unit, and can be blended to make a high-efficiency green OLED. F8BT calso used as an acceptor for polymer blends devices.
General Information
| CAS Number | 196207-58-6 |
| Chemical Formula | C41H64B2O4 |
| Molecular Weight | 642.57 g/mol |
| Synonyms |
|
| Classification / Family | Fluorene, Boronic esters, Organic semiconducting materials, Semiconductor synthesis, Low band gap polymers, OFETs, OLED, Organic photovoltaics, Polymer solar cells |
Chemical Structure
Product Details
| Purity | >98% |
| Melting Point | 127 °C - 131 °C |
| Appearance | White powder |
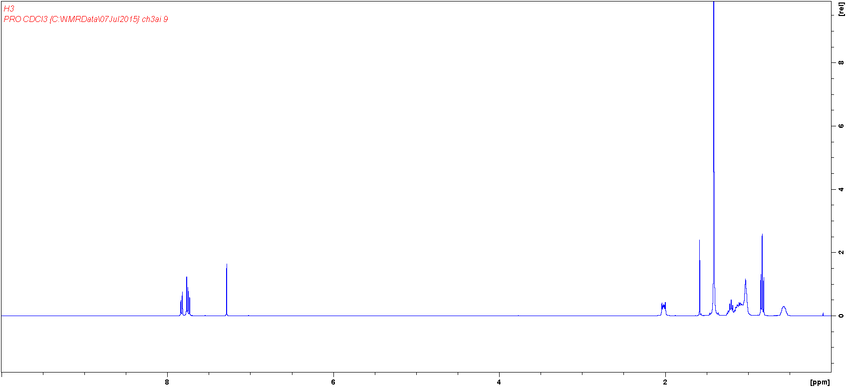
Characterization
MSDS Documentation
Literature and Reviews
- Recent progress of high performance polymer OLED and OPV materials for organic printed electronics, C. Sekine et al., Sci. Technol. Adv. Mater., 15, 034203 (2014)
- Morphological study of F8BT:PFB thin film blends, M. Abdullaa et al., Org. Electronics, 23, 87-98 (2015)
- Highly Efficient Inverted Polymer Solar Cells Based on a Cross-linkable Water-/Alcohol-Soluble Conjugated Polymer Interlayer, K. Zhang et al., ACS Appl. Mater. Interfaces, 6 (13), 10429-10435 (2014)
