3,3′,5,5′-Tetrabromo-2,2′-bithiophene
CAS Number 125143-53-5
Chemistry Building Blocks, Heterocyclic Building Blocks, Materials, Monomers3,3′,5,5′-Tetrabromo-2,2′-bithiophene
This intermediate is widely used for the synthesis of semiconducting molecules, oligomers and conjugated polymers and more fused aromatic rings.
Specifications | MSDS | Literature and Reviews
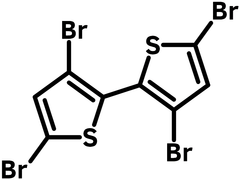
3,3′,5,5′-Tetrabromo-2,2′-bithiophene (CAS number 125143-53-5) has a structure of two thiophenes joined at 2,2'-positions and each is brominated with two bromides at 3,5-positions. This tetra-brominated compound adds functions for further polymerization, cyclization to form polymers or to add conjugation to the bithiophene unit to form fused rings.
3,3′,5,5′-Tetrabromo-2,2′-bithiophene has been used for the synthesis of dithieno[3,2-b:2′,3′-d]pyrrole, dithieno[3,2-b:2,3-d]silole and their derivatives. Bromides at 3,3'-positions give rise to obtaining fused rings for higher degree of conjugation and flat structures. Polymerization of 3,3′,5,5′-tetrabromo-2,2′-bithiophene and ethynylbenzene monomers via the palladium-catalyzed Sonogashira–Hagihara cross-coupling reaction gives microporous polymers that show ultrahigh absorption performance for iodine vapor with an uptake of up to 345 wt%. Homopolymer poly(N-(2-octyldodecyl)-2,2′-bithiophene-3,3′-dicarboximide), derived from 3,3′,5,5′-tetrabromo-2,2′-bithiophene, exhibits n-channel FET activity and its films exhibit a very high degree of crystallinity and an electron mobility >0.01 cm2 V-1s-1 with a current on−off ratio of 107.
3,3′,5,5′-Tetrabromo-2,2′-bithiophene can be obtained by direct bromination of 2,2'-bithiophene with bromine in chloroform and acetic acid. N-Bromosuccinimide (NBS) can also be used as the oxidizing agent to replace bromine for the synthesis. By refluxing 3,3′,5,5′-Tetrabromo-2,2′-bithiophene with zinc in hydrogen chloride and acetic acid, it gives 3,3'-dibromobithiophene.
Bithiophene building block
for the synthesis of OLED and organic photovoltaic materials
Worldwide shipping
Quick and reliable shipping
Capped with bromide
for facil coupling reactions
High purity
>98% Purity
General Information
| CAS Number | 125143-53-5 |
| Chemical Formula | C8H2Br4S2 |
| Full Name | 3,3′,5,5′-Tetrabromo-2,2′-bithiophene |
| Molecular Weight | 481.85 g/mol |
| Synonyms | 3,5-dibromo-2-(3,5-dibromo-2-thienyl)thiophene |
| Classification / Family | Bithiophene, semiconductor synthesis intermediates, low band gap polymers, OLED, OFETs, organic photovoltaics |
Chemical Structure
Product Details
| Purity | >98% (1H NMR in CDCl3) |
| Melting Point | 144.0 °C |
| Appearance | Light yellow to white/off white powder/crystals |
MSDS Documentation
3,3′,5,5′-Tetrabromo-2,2′-bithiophene MSDS Sheet
Literature and Reviews
- Synthesis and Properties of Alternating Copolymers Based on 4H-Cyclopenta[2,1-b:3,4-b']dithiophene and 4H-Dithieno[3,2-b:2',3'-d]silol, F. Drozdov et al., Polym. Sci. Ser. B 61, 56–76 (2019); DOI: 10.1134/S1560090419010032.
- Novel thiophene-bearing conjugated microporous polymer honeycomb-like porous spheres with ultrahigh iodine uptake, F. Ren et al., Chem. Commun., 52, 9797-9800 (2016); DOI: 10.1039/c6cc05188j.
- Two-photon absorption chromophores with a tunable [2,2']bithiophene core, C. Chou et al., Tetrahedron, 62, 8467–8473 (2006); DOI: 10.1016/j.tet.2006.06.085.
