Br-BT-CHO
CAS Number 1071224-34-4
Carbaldehyde Monomers, Chemistry Building Blocks, Heterocyclic Building Blocks, Materials,Specifications | MSDS | Literature and Reviews
Br-BT-CHO (CAS number 1071224-34-4), namely 7-Bromobenzo[c][1,2,5]thiadiazole-4-carbaldehyde, is a benzothiadiazole derivative with both bromine and aldehyde functional groups. Br-BT-CHO can be used as intermediate for further either carbon-carbon single bond (C-C) via Stille coupling, or double bond (C=C) formation via Aldol condensation reactions.
Br-BT-CHO as an intermediate can be used for the synthesis of non-fullerene acceptors such as o-IDTBR and FBR.
Benzothiadiazole derivative
For the synthesis of NFAs
High purity
>98% Br-BT-CHO Purity
Worldwide shipping
Quick and reliable shipping
Substituted with bromide and aldehyde
For muti-step reactions
General Information
| CAS Number | 1071224-34-4 |
| Chemical Formula | C7H3BrN2OS |
| Molecular Weight | 243.08 g/mol |
| Full Name | 7-Bromobenzo[c][1,2,5]thiadiazole-4-carbaldehyde |
| Synonyms | 7-Bromo-4-formyl-2,1,3-benzothiadiazole |
| Classification / Family | Benzothiadiazole, Monomer and intermediates, None-fullerene acceptors (NFAs), NFA-OSCs, printing electronics |
Chemical Structure
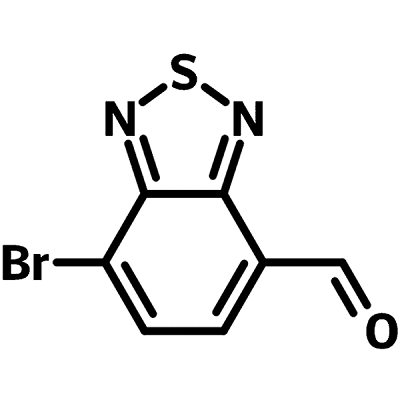
Product Details
| Purity | >98% (by NMR) |
| Melting Point | 192 °C - 194 °C |
| Appearance | Slightly yellow crystalline powder |
MSDS Documentation
Literature and Reviews
- P3HT-Based Polymer Solar Cells with 8.25% Efficiency Enabled by a Matched Molecular Acceptor and Smart Green-Solvent Processing Technology, X. Xu et al., Adv. Mater., 31 (52), 1906045 (2019); DOI: 10.1002/adma.201906045.
- π-Bridge-Independent 2-(Benzo[c][1,2,5]thiadiazol-4-ylmethylene)malononitrile-Substituted Nonfullerene Acceptors for Efficient Solar Cells, K. Wang et al., Chem. Mater., 28 (7), 2200–2208 (2016); DOI: 10.1021/acs.chemmater.6b00131.
- Significant Improvement of Dye-Sensitized Solar Cell Performance by Small Structural Modification in π-Conjugated Donor–Acceptor Dyes, S. Haid et al., Adv. Funct. Mater., 22, 1291–1302 (2012); DOI: 10.1002/adfm.201102519.
