4CzIPN-Ph
CAS Number 1469705-37-0
Dopant Materials, Materials, OLED Materials, Red Dopant Materials,A TADF orange emitter
Highly efficient TADF materials for OLEDs, Photosynthesis, Time-Resolved Luminescence Imaging and Sensing
Specifications | MSDS | Literature and Reviews
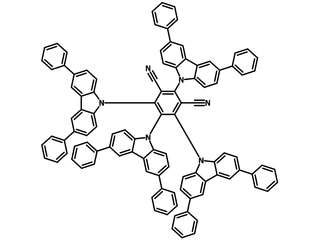
4CzIPN-Ph (CAS number 1469705-37-0), also known as 2,4,5,6-tetrakis(3,6-diphenylcarbazol-9-yl)-1,3-dicyanobenzene, has the structure origin of 4CzIPN with 8 phenyl substituents at 3,6-positions of carbazolyl moieties. Due to the steric conformational effects, the phenyl rings are relatively decoupled from the core of the molecule in 4CzIPN-Ph.
Wavefunctions suggests a partial delocalization of the hole electronic density into the outer phenyl rings, thus enhancing its charge transfer character with reduces ΔEST. Devices using single DMAC-DPS: 4CzTPN-Ph emission layer achieved a maximum external quantum efficiency of 13.4%, maximum power efficiency of 38.3 lm W-1 and current-insensitive Commission Internationale de I'Eclairage (CIE) coordinates of (0.29, 0.39).
4CzIPN-Ph, 4CzPN-Ph and 4CzTPN-Ph are isomers with two nitrile (CN) groups sitting at meta-, ortho- and para- positions of the benzene ring respectively.
General Information
| CAS Number | 1469705-37-0 |
| Full Name | 2,4,5,6-Tetrakis(3,6-diphenylcarbazol-9-yl)-1,3-dicyanobenzene |
| Synonyms | 2,4,5,6-Tetrakis(3,6-diphenyl-9H-carbazol-9-yl)-1,3-benzenedicarbonitrile, 2,4,5,6-Tetrakis(3,6-diphenyl-9H-carbazol-9-yl)isophthalonitrile |
| Chemical Formula | C104H64N6 |
| Molecular Weight | 1397.66 g/mol |
| Absorption* | λmax 406 nm in film |
| Photoluminescence | λem 598 nm in film |
| HOMO/LUMO | HOMO = 5.48 eV, LUMO = 2.66 eV [1] |
| Classification / Family | Carbazole derivatives, Isophthalonitrile TADF materials, Orange dopant materials, Sublimed materials |
Chemical Structure
Product Details
| Purity | Unsublimed >98.0% (1H NMR) |
| Melting Point | N/A |
| Appearance | Orange powder/crystals |
MSDS Documentation
Literature and Reviews
- Simple-structure organic light emitting diodes: Exploring the use of thermally activated delayed fluorescence host and guest materials, Z. Liu et al., Org. Electron., 41, 237-244 (2017); DOI: 10.1016/j.orgel.2016.11.010.
-
New Triplet Sensitization Routes for Photon Upconversion: Thermally Activated Delayed Fluorescence Molecules, Inorganic Nanocrystals, and Singlet-to-Triplet Absorption, N. Yanai et al., Acc. Chem. Res., 50 (10), 2487–2495 (2017); DOI: 10.1021/acs.accounts.7b00235.
-
Organic Thermally Activated Delayed Fluorescence Materials for Time-Resolved Luminescence Imaging and Sensing, F. Ni et al., Adv. Opt. Mater., 8 (14), 1902187 (2020); DOI: 10.1002/adom.201902187.
