DMAC-BP
CAS Number 1685287-55-1
Dopant Materials, Green Dopant Materials, High Purity Sublimed Materials, Materials,DMAC-BP, green TADF emitter
High-purity and available online for priority dispatch, Bis[4-(9,9-dimethyl-9,10-dihydroacridine)phenyl]methanone, CAS No. 1685287-55-1, Sublimed ≥99.0%
DMAC-BP, bis[4-(9,9-dimethyl-9,10-dihydroacridine)phenyl]methanone is better known as a green Thermally Activated Delayed Fluorescence (TADF) emitter.
Due to the short conjugation length of the DMAC and BP group, a higher energy of the lowest locally-excited triplet state (3LE) is achieved. This energy is almost equal to that of the triplet charge-transfer state (3CT). The difference of the lowest singlet and triplet excited states ΔEST is 0.07 eV [2].
![]()
DMAC-BP from Ossila was used in the high-impact paper (IF 29.4), Single-Layer Organic Light-Emitting Diode with Trap-Free Host Beats Power Efficiency and Lifetime of Multilayer Devices,O. Sachnik et al., Adv. Mater., 36 (16), 2311892 (2024); DOI: 10.1002/adma.202311892.
Compared to mCP-doped films, neat films of DMAC–BP have a slightly red-shifted emission band with a maximum of 506 nm, a slightly lower photoluminescence quantum yield (PLQY) of 0.85, and TADF lifetime of 2.7 μs.
General Information
| CAS number | 1685287-55-1 |
|---|---|
| Chemical formula | C43H36N2O |
| Molecular weight | 596.76 g/mol |
| Absorption | n/a |
| Fluorescence | λem 506 nm (in film) |
| HOMO/LUMO | HOMO = -5.8 eV, LUMO = -3.1 [1] |
| Synonyms | Bis[4-(9,9-dimethyl-9,10-dihydroacridine)phenyl]methanone |
| Classification / Family | Electron transport layer (ETL) materials, Solution-processed OLED materials, TADF green emitter materials, PHOLEDs, Sublimed materials |
Product Details
| Purity | >99.0% (sublimed) |
|---|---|
| Melting Point | TGA Td = 410 oC (5% weight loss) |
| Appearance | Light yellow powder/crystals |
*Sublimation is a technique used to obtain ultra pure-grade chemicals. For more details about sublimation, please refer to the Sublimed Materials.
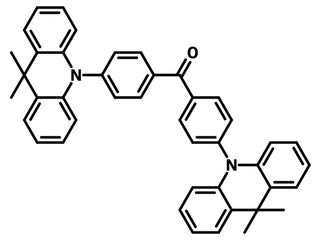
Chemical Structure
Device Structure(s)
| Device structure | ITO/HATCN (5 nm)/NPB (40 nm)/TCTA (10 nm)/DMAC-BP:DMIC-TRZ (30 nm)/B3PyMPM (40 nm)/LiF (1 nm)/Al (150 nm) [1] |
|---|---|
| Color | Green |
| Max. Power Efficiency | 52.9 lm W−1 |
| Max. EQE | 21% |
| Device structure | ITO/HATCN (5 nm)/NPB (40 nm)/TCTA (10 nm)/DMAC-BP:26DCzPPy (30 nm)/B3PyMPM (40 nm)/LiF (1 nm)/Al (150 nm) [1] |
|---|---|
| Color | Green |
| >Max. Power Efficiency | 38.4 lm W−1 |
| Max. EQE | 19% |
| Device structure | ITO/MoO3 (1 nm)/DMAC-BP (60 nm)/TPBi (60 nm)/LiF (1 nm)/Al [2] |
|---|---|
| Color | Green |
| Max. Luminance | 51,100 cd/m2 |
| Power Efficiency@100 cd/m2 | 25 lm W−1 |
| Max. EQE | 10.6% |
| Device structure | ITO/MoO3 (1 nm)/mCP (40 nm)/DMAC-BP (30 nm)/TPBi (50 nm)/LiF (1 nm)/Al [2] |
|---|---|
| Color | Green |
| Max. Luminance | 45,300 cd/m2 |
| Power Efficiency@100 cd/m2 | 59 lm W−1 |
| Max. EQE | 18.9% |
MSDS Documentation
Literature and Reviews
- Highly Efficient Full-Color Thermally Activated Delayed Fluorescent Organic Light-Emitting Diodes: Extremely Low Efficiency Roll-Off Utilizing a Host with Small Singlet–Triplet Splitting, D. Zhang et al., ACS Appl. Mater. Interfaces, 9 (5), 4769–4777 (2017); DOI: 10.1021/acsami.6b15272.
- Nearly 100% Internal Quantum Effi ciency in Undoped Electroluminescent Devices Employing Pure Organic Emitters, Q, Zhang et al., Adv. Mater., 27, 2096–2100 (2015); DOI: 10.1002/adma.201405474.
- Undoped highly efficient green and white TADF-OLEDs developed by DMAC-BP: manufacturing available via interface engineering, X. Jiang et al., J Mater Sci: Mater Electron (2020). DOI: 0.1007/s10854-020-04450-z.
