mCBP
CAS Number 342638-54-4
Fluorescent Host Materials, High Purity Sublimed Materials, Host Materials, Materials,mCBP, host material for blue, green, orange, and yellow fluorescent and phosphorescent emitters
Applications in OLED and TADF-OLED devices, now available online for priority dispatch, 3,3′-Di(9H-carbazol-9-yl)-1,1′-biphenyl, CAS No. 342638-54-4, Sublimed ≥99.0%, Unsublimed ≥98.0%
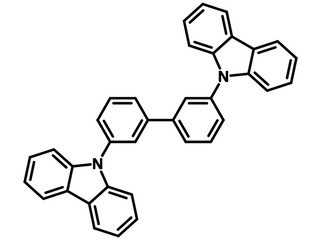
mCBP, 3,3′-Di(9H-carbazol-9-yl)-1,1′-biphenyl, is an isomer of CBP, 4,4′-Di(9H-carbazol-9-yl)-1,1′-biphenyl. The meta-linkage in mCBP limits conjugation to the central biphenyl, preventing excimer formation and thus resulting in a higher triplet energy of 2.8 eV.
Like CBP and CDBP, mCBP is widely used in OLED and TADF-OLED devices as a host material for blue, green, orange, and yellow fluorescent and phosphorescent emitters.
With two carbazole units, mCBP is electron-rich and can be used to form exciplexes with electron acceptors (such as POT2T) as blue emitters.
General Information
| CAS number | 342638-54-4 |
|---|---|
| Full name | 3,3′-Di(9H-carbazol-9-yl)-1,1′-biphenyl |
| Chemical formula | C36H24N2 |
| Molecular weight | 484.59 g/mol |
| Absorption | λmax 340 nm in toluene |
| Fluorescence | n/a |
| HOMO/LUMO | HOMO = 6.0 eV, LUMO = 2.4 eV [1] |
| Synonyms | n/a |
| Classification / Family | Carbazole derivatives, fluorescent host materials, blue exciplex host materials, sublimed materials, OLED-TADF, Organic electronics. |
* Measurable with a USB spectrometer
Product Details
| Purity |
Sublimed* >99% (HPLC), Unsublimed >98% (1H NMR) |
|---|---|
| Melting point | n/a |
| Appearance | White crystals/powder |
* Sublimation is a technique used to obtain ultra pure-grade chemicals, see sublimed materials for more sublimed materials.
Chemical Structure
Device Structure(s)
| Device structure | ITO/ZnO (20 nm)/10 wt% Cs2CO3:BPhen (20 nm)/BPhen (20 nm)/10 wt% TXO-PhCz:mCBP (30 nm)/TAPC (40 nm)/MoO3 (10 nm)/Al (100 nm) [1] |
|---|---|
| Color | Green |
| Max. Power Efficiency | 35.6 lm W−1 |
| Max. Current Efficiency | 53.9 cd/A |
| Max. EQE | 16.4% |
| Device structure | ITO/HATCN (15 nm)/TAPC (60 nm)/TCTA (5 nm)/mCBP (5 nm)/mCBP:POT2T:Ir(tptpy)2acac (1:1:0.2%, 15 nm)/POT2T (45 nm)/Liq (1.5 nm)/Al (150 nm) [2] |
|---|---|
| Color | White |
| Max. Power Efficiency | 97.1 lm W−1 |
| Max. Current Efficiency | 74.2 cd/A |
| Max. EQE | 22.45% |
| Device structure | ITO (50 nm)/NPD (40 nm)/TCTA (15 nm)/mCP) (15 nm)/1 wt% DABNA-2*:mCBP (20 nm)/TSPO1* (40 nm)/LiF (1 nm)/Al (100 nm) [3] |
|---|---|
| Color | Blue |
| Max. Power Efficiency | 15.1 lm W−1 |
| Max. Current Efficiency | 21.1 cd/A |
| Max. EQE | 20.2% |
| Device structure | ITO/HATCN (10 nm)/NPD (30 nm)/TAPC (10 nm)/ 2 % Pt7O7*:mCBP (25 nm)/DPPS (10 nm)/BmPyPB (40 nm)/LiF/Al [4] |
|---|---|
| Color | Blue |
| Max. Power Efficiency | 32.4 lm W−1 |
| Max. EQE | 26.3% |
| Device structure | ITO/HATCN (10 nm)/NPD (30 nm)/TAPC (10 nm)/ 18 % Pt7O7*:mCBP (25 nm)/DPPS (10 nm)/BmPyPB (40 nm)/LiF/Al [4] |
|---|---|
| Color | White |
| Max. Power Efficiency | 56.7 lm W−1 |
| Max. EQE | 24.1% |
| Device structure | ITO (50 nm)/NPD (40 nm)/TCTA (15 nm)/mCP) (15 nm)/1 wt% DABNA-2*:mCBP (20 nm)/TSPO1* (40 nm)/LiF (1 nm)/Al (100 nm) [5] |
|---|---|
| Color | Yellow |
| Max. Power Efficiency | 56.2 lm W−1 |
| Max. Current Efficiency | 66.2 cd/A |
| Max. EQE | 23.2% |
| Device structure | ITO/TAPC (35 nm)/1 wt%-TBRb:25 wt%-PXZ-TRX*:mCBP (30 nm)/T2T (10 nm)/Alq3 (55 nm)/LiF (0.8 nm)/Al (100 nm) [6] |
|---|---|
| Color | Yellow |
| Max. Power Efficiency | 33.0 lm W−1 |
| Max. Current Efficiency | 56.0 cd/A |
| Max. EQE | 17.2% |
*For chemical structure information, please refer to the cited references
Pricing
| Grade | Order Code | Quantity | Price |
|---|---|---|---|
| Sublimed (>99% purity) | M2186A1 | 500 mg | £260 |
| Sublimed (>99% purity) | M2186A1 | 1 g | £400 |
| Sublimed (>99% purity) | M2186A1 | 5 g | £1650 |
| Unsublimed (>98% purity) | M2186B1 | 1 g | £200 |
| Unsublimed (>98% purity) | M2186B1 | 5 g | £800 |
MSDS Documentation
Literature and Reviews
- n-Doping-induced efficient electron-injection for high efficiency inverted organic light-emitting diodes based on thermally activated delayed fluorescence emitter, Y. Chen et al., J. Mater. Chem. C, 5, 8400 (2017); DOI: 10.1039/c7tc02406a.
- High efficiency (~ 100 lm W-1) hybrid WOLEDs by simply introducing ultrathin non-doped phosphorescent emitters in a blue exciplex host, S, Ying et al., J. Mater. Chem. C, 6, 7070 (2018); DOI: 10.1039/c8tc01736k.
- Ultrapure Blue Thermally Activated Delayed Fluorescence Molecules: Efficient HOMO–LUMO Separation by the Multiple Resonance Effect, T. Hatakeyama et al., Adv. Mater., 28, 2777–2781 (2016); DOI: 10.1002/adma.201505491.
