4CzTPN-Ph
CAS Number 1416881-55-4
Dopant Materials, High Purity Sublimed Materials, Materials, OLED Materials,4CzTPN-Ph, orange emitting material used in highly efficient TADF-OLED devices
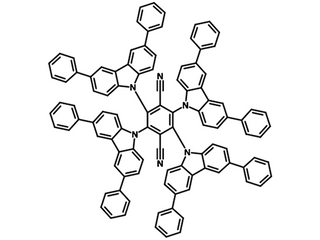
Used to effectively prevent molecular interactions, 2,3,5,6-tetrakis(3,6-diphenylcarbazol-9-yl)-1,4-dicyanobenzene, CAS No. 1416881-55-4
4CzTPN-Ph (CAS number 1416881-55-4), a well-known orange emitting material that is widely used in highly efficient TADF-OLED devices.
Comparing to 4CzTPN, the eight phenyl groups at 3,6-positions of carbazole moieties can further increase the electron-donating ability for 4CzTPN-Ph while at the same time greater steric hindrance is introduced. The increased steric hindrance can effectively prevent molecular interactions, i.e. the formation of the excimer.
With the donor-acceptor structure enabling the molecule with good two emission characteristics, 4CzTPN-Ph can also be used for both one and two-photon cellular fluorescence imaging as nanoparticles dispersed in water with good dispersibility, superior resistance against photodegradation and photobleaching and low cytotoxicity.
General Information
| CAS number | 1416881-55-4 |
|---|---|
| Full name | 2,3,5,6-tetrakis(3,6-diphenylcarbazol-9-yl)-1,4-dicyanobenzene |
| Synonyms | 2,3,5,6-tetrakis(3,6-diphenyl-9H-carbazol-9-yl)terephthalonitrile |
| Chemical formula | C104H64N6 |
| Molecular weight | 1397.66 g/mol |
| Absorption | λmax 377 nm, 547 nm in toluene |
| Fluorescence | λem 577 nm in toluene |
| HOMO/LUMO | HOMO = 5.90 eV, LUMO = 4.0 eV, T1 = 2.21 eV [1] |
| Classification / Family | Carbazole, TADF materials, Orange dopant materials, Sublimed materials |
* Measurable with an optical spectrometer
Product Details
| Purity | Unsublimed >98%; Sublimed >99.0% (1H NMR) |
|---|---|
| Melting point | n.a. |
| Appearance | Orange powder/crystals |
* Sublimation is a technique used to obtain ultra pure-grade chemicals, see sublimed materials.
Chemical Structure
Device Structure(s)
| Device structure | ITO/MoO3 (3 nm)/mCP (20 nm)/mCBP:PO-T2T* (20 nm)/PO-T2T:5.0 wt.% 4CzTPN-Ph (10 nm)/ PO-T2T (40 nm)/LiF (0.8 nm)/Al [2] |
|---|---|
| Color | White |
| Max. Current Efficiency | 11.88 cd/A |
| Max. EQE | 5.75% |
| Max. Power Efficiency | 9.33 Im/W |
| Device structure | ITO/MoO3 (5 nm)/mCP (40 nm)/DMAC-DPS: 0.4 wt.% 4CzTPN-Ph (30 nm)/SPPO13* (50 nm)/CsF (1 nm)/Al (150 nm) [3] |
|---|---|
| Color | White |
| Max. EQE | 14.7% |
| Max. Power Efficiency | 35.6 Im/W |
| Device structure | ITO/MoO3 (5 nm)/mCP (40 nm)/DMAC-DPS: 9 wt.% 4CzTPN-Ph (30 nm)/SPPO13 (50 nm)/CsF (1 nm)/Al (150 nm) [3] |
|---|---|
| Color | Orange |
| Max. EQE | 11.0% |
| Max. Power Efficiency | 36.6 Im/W |
| Device structure | ITO/HATCN (10 nm)/Tris-PCz (35 nm)/10 wt.% 4CzPN:mCBP (G-EML) (5 nm)/6 wt.% 4CzPN:2 wt.% 4CzTPN-Ph:mCBP (R-EML) (4 nm)/10 wt.% 3CzTRZ:PPT (B-EML) (6 nm)/PPT (50 nm)/LiF (0.8 nm)/Al (100 nm) [4] |
|---|---|
| Color | White |
| Max. Power Efficiency | 34.1 lm W−1 |
| Max. Current Efficiency | 45.6 cd/A |
| Max. EQE | 17.0% |
| Device structure | ITO/HATCN (10 nm)/Tris-PCz (35 nm)/10 wt. % 4CzPN:mCBP (G-EML) (5 nm)/6 wt. % 4CzPN:2 wt. % 4CzTPN-Ph:mCBP (R-EML) (4 nm)/10 wt. % 3CzTRZ:PPT (B-EML) (6 nm)/PPT (50 nm)/LiF (0.8 nm)/Al (100 nm) [4] |
|---|---|
| Color | White |
| Max. Current Efficiency | 13.13 cd/A |
| Max. EQE | 6.8% |
| Max. Power Efficiency | 4.75 lm W-1 |
*For chemical structure information, please refer to the cited references.
MSDS Documentation
Literature and Reviews
- Guest concentration, bias current, and temperature-dependent sign inversion of magneto-electroluminescence in thermally activated delayed fluorescence devices, J. Deng et al., Sci. Rep., 7:44396 (2017); DOI: 10.1038/srep44396.
- Simple structured hybrid WOLEDs based on incomplete energy transfer mechanism: from blue exciplex to orange dopant, T. Zhang et al., Sci. Rep., 5:10234 (2015); DOI: 10.1038/srep10234.
- Simple-structure organic light emitting diodes: Exploring the use of thermally activated delayed fluorescence host and guest materials, Z. Liu et al., Org. Electron., 41, 237-244 (2017); doi: 0.1016/j.orgel.2016.11.010.
