DBP
CAS Number 175606-05-0
Dopant Materials, High Purity Sublimed Materials, Materials, OLED Materials,DBP, promising organic small-molecule semiconductor
Electron donor and acceptor for highly efficient photovoltaic and OLED applications, 5,10,15,20-Tetraphenylbisbenz[5,6]indeno[1,2,3-cd:1′,2′,3′-lm]perylene, CAS No. 175606-05-0, Sublimed ≥99.0%
5,10,15,20-Tetraphenylbisbenz[5,6]indeno[1,2,3-cd:1′,2′,3′-lm]perylene (DBP), also known as tetraphenyldibenzoperiflanthene, is a promising organic small-molecule semiconductor. It can be used as either an electron donor or acceptor for highly efficient photovoltaic and OLED applications.
With perylene as an electron-rich core and extended conjugations, DBP can also be used in photovoltaic light-emitting diodes (PVOLEDs) devices as an electron-donating layer (EDL) material.
General Information
| CAS number | 175606-05-0 |
|---|---|
| Full name | 5,10,15,20-Tetraphenylbisbenz[5,6]indeno[1,2,3-cd:1′,2′,3′-lm]perylene |
| Chemical formula | C64H36 |
| Molecular weight | 804.97 g/mol |
| Absorption* | λmax 333 nm in THF |
| Fluorescence | λem 610 nm in THF |
| HOMO/LUMO | HOMO = 5.5 eV, LUMO = 3.5 eV [1] |
| Synonyms | Tetraphenyldibenzoperiflanthene, Dibenzo{[f,f′]-4,4′,7,7′-tetraphenyl}diindeno[1,2,3-cd:1′,2′,3′-lm]perylene, Red 2 |
| Classification / Family | Perylene derivatives, Hydrocarbons, Red dopant TADF materials, Phosphorescent organic light-emitting devices (PHOLEDs), Photovoltaics, Sublimed materials |
* Measurable with an optical spectrometer
Product Details
| Purity | Sublimed* >99.0% (HPLC) |
|---|---|
| Melting point | TGA: >350 °C (0.5% weight loss) |
| Appearance | Red powder/crystals |
*Sublimation is a technique used to obtain ultra pure-grade chemicals. For more details about sublimation, please refer to the Sublimed Materials.
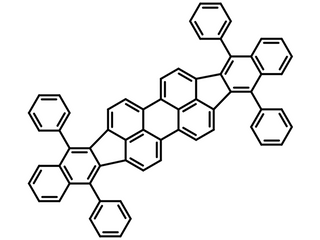
Chemical Structure
Device Structure(s)
| Device structure | ITO/α-NPD (30 nm) /DPEPO (10 nm)/TPBi (40 nm)/1 wt% DBP:10 wt% TTPA:mCP (8 nm)/mCP (2 nm)/DMACDPS (7.5 nm)/LiF (0.5 nm)/Al (100 nm) [1] |
|---|---|
| Color | White |
| Max. EQE | 12.1% |
| Max. Power Efficiency | 22 Im/W |
| Device structure | ITO/CFx/NPB (60 nm)/ Rubrene+ 1% DBP (40 nm)/DBzA (20 nm)/LiF (1 nm)/Al [2] |
|---|---|
| Color | Red |
| Current Efficiency@ 20 mA/cm2 | 5.4 cd/A |
| EQE@ 20 mA/cm2 | 4.7% |
| Power Efficiency@ 20 mA/cm2 | 5.3 Im/W |
| Device structure | ITO (95 nm)/ HATCN (5 nm)/TAPC (20 nm)/CBP: 7 wt% NI-1-PhTPA (10 nm)/CBP (3 nm)/CBP: 3 wt% PXZDSO2 (5 nm)/CBP: 5 wt% PXZDSO2: 0.3 wt% DBP (5 nm)/CBP: 3 wt% PXZDSO2 (5 nm)/CBP (3 nm)/CBP: 7 wt% NI-1-PhTPA (10 nm)/TmPyPB (55 nm)/LiF (1 nm)/Al (100 nm) [3] |
|---|---|
| Color | White |
| Max. Current Efficiency | 51.4 cd/A |
| Max. EQE | 19.2% |
| Max. Power Efficiency | 47.5 Im/W |
| Device structure | ITO (95 nm)/ HATCN (5 nm)/TAPC (20 nm)/ CBP: 10 wt% NI-1-PhTPA (10 nm)/CBP (3 nm)/CBP: 3 wt% PXZDSO2 (5 nm)/CBP: 5 wt% PXZDSO2: 0.35 wt% DBP (5 nm)/ CBP: 3 wt% PXZDSO2 (5 nm)/CBP (3 nm)/CBP: 10 wt% NI-1-PhTPA (10 nm);/TmPyPB (55 nm)/LiF (1 nm)/Al (100 nm) [3] |
|---|---|
| Color | White |
| Max. Current Efficiency | 42.2cd/A |
| Max. EQE | 17.3% |
| Max. Power Efficiency | 38.4 Im/W |
| Device structure | ITO (95 nm)/PEDOT:PSS (40 nm)/2 wt% DBP:15 wt% DC-TC*:CBP (40 nm)/TmPyPB (50 nm)/LiF (1 nm)/Al (100 nm) [4] |
|---|---|
| Color | Red |
| Current Efficiency@ 100 mA/cm2 | 10.15 cd/A |
| EQE@ 100 mA/cm2 | 6.65% |
*For chemical structure information, please refer to the cited references.
MSDS Documentation
Literature and Reviews
- High-Efficiency White Organic Light-Emitting Diodes Based on a Blue Thermally Activated Delayed Fluorescent Emitter Combined with Green and Red Fluorescent Emitters, T. Higuchi et al., Adv. Mater., 27, 2019–2023 (2015); DOI: 10.1002/adma.201404967.
- High efficiency red organic light-emitting devices using tetraphenyldibenzoperiflanthene-doped rubrene as an emitting layer, K. Okumoto et al., Appl. Phys. Lett. 89, 013502 (2006); doi: 10.1063/1.2218833.
- High-Efficiency WOLEDs with High Color-Rendering Index based on a Chromaticity-Adjustable Yellow Thermally Activated Delayed Fluorescence Emitter, X. Li et al., Adv. Mater., 28, 4614–4619 (2016); DOI: 10.1002/adma.201505963.
