4CzIPN-Me
CAS Number 1469703-61-4
Dopant Materials, Green Dopant Materials, Materials, OLED Materials,A TADF yellow emitter
Used either as solo emitter in highly efficient TADF-OLEDs or an assistant dopant for TAF-OLEDs, 2,4,5,6-Tetra(3,6-dimethylcarbazol-9-yl)-1,3-dicyanobenzene, CAS No. 1469703-61-4, m4CzIPN
Specifications | MSDS | Literature and Reviews
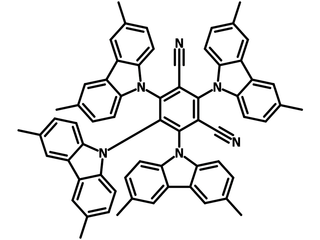
4CzIPN-Me, namely 2,4,5,6-Tetra(3,6-dimethylcarbazol-9-yl)-1,3-dicyanobenzene, is the methyl substituted 4CzIPN at 3,6-positions of the four carbazolyl units. Methyl groups at 3,6-positions will enhance the stability but also the solubility of the Carbazolyl benzonitriles. 4CzIPN-Me is the isomeric structure to 4CzPN-Me and 4CzTPN-Me.
By introducing an assistant dopant having a rather large kRISC~106, 4CzIPN-Me, both high electroluminescence efficiency and enhanced operational stability could be achieved in TADF assisted fluorescence (TAF-OLEDs). The state-of-the-art TAF-OLED technology uses a TADF molecule as an assistant dopant and a fluorescent molecule as an end emitter in a host matrix. The suppression of exciton annihilation was demonstrated via efficient and rapid triplet- singlet up conversion.
Electrogenerated chemiluminescence (ECL) study shows that the ECL intensity of 4CzIPN-Me decreases during voltage cycles due to the fact that polymerization happens on the carbazolyl moieties.
General Information
| CAS Number | 1469703-61-4 |
| Full Name | 2,4,5,6-Tetra(3,6-dimethylcarbazol-9-yl)-1,3-dicyanobenzene |
| Synonyms | m4CzIPN, 2,4,5,6-Tetrakis(3,6-dimethyl-9H-carbazol-9-yl)-1,3-benzenedicarbonitrile, 2,4,5,6-tetrakis(3,6-dimethyl-9H-carbazol-9-yl)isophthalonitrile |
| Chemical Formula | C64H48N6 |
| Molecular Weight | 901.11 g/mol |
| HOMO/LUMO | HOMO = 5.90 eV, LUMO = 2.81 eV [1] |
| Classification / Family | Carbazole derivatives, Isophthalonitriles, TADF materials, Yellow dopant materials, Sublimed materials |
Chemical Structure
Product Details
| Purity | Unsublimed >98.0% (1H NMR) |
| Melting Point | N/A |
| Appearance | Orange powder/crystals |
Device Structure(s)
| Device Structure | ITO (100 nm)/HAT-CN (10 nm)/TAPC (30 nm)/6.3mol%-4CzIPN-Me:mCBP (30 nm)/T2T (10 nm)/Alq3 (55 nm)/LiF (0.8 nm)/Al (100 nm) [2] |
| Color |
|
| Max. Current Efficiency | 73 cd/A |
| Max. EQE | 21% |
| LT50@1,000 cd/m2 | 1472 h |
| Device Structure | ITO (100 nm)/HAT-CN (10 nm)/TAPC (30 nm)/0.65mol%-TBRb:6.3mol%-4CzIPN-Me:mCBP (30 nm)/T2T (10 nm)/Alq3 (55 nm)/LiF (0.8 nm)/Al (100 nm) [2] |
| Color |
|
| Max. Current Efficiency | 62 cd/A |
| Max. EQE | 19.1% |
| LT50@1,000 cd/m2 | 3775 h |
MSDS Documentation
Literature and Reviews
- Efficiency loss processes in hyperfluorescent OLEDs: A kinetic Monte Carlo study, S. Gottardi et al., Appl. Phys. Lett., 114, 073301 (2019); DOI: 10.1063/1.5079642.
- Dual enhancement of electroluminescence efficiency and operational stability by rapid upconversion of triplet excitons in OLEDs, T. Furukawa et al., Sci Rep., 5, 8429 (2015); DOI: 10.1038/srep08429.
- High Efficiency in a Solution-Processed Thermally Activated Delayed-Fluorescence Device Using a Delayed-Fluorescence Emitting Material with Improved Solubility, Y. Cho et al., Adv. Mater., 26 (38), 6642-6646 (2014); DOI: 10.1002/adma.201402188.
